Drug/Ligand/Receptor Interactions
Surface Plasmon Resonance (SPR)
SPR arises when photons are reflected from a conducting film at the interface between two media of different refractive indices. This phenomenon can be utilized by measuring a change in the angle of reflection of polarized near infra-red light on a gold surface, which occurs when the index of refraction of the surface changes. The Biacore 8K instrument is used to measure biomolecular interactions, specifically the association rate and dissociation rate of analytes from a biomolecule immobilized on one side of a gold film. In the instrument, light is reflected on the opposite side of a gold film to excite the surface plasmons, that is, the oscillations of free electrons propagating along the film’s surface. The angle of reflection of polarized light reflecting off this surface is measured. When the immobilized biomolecules are bound by their ligands, an alteration in surface plasmons on the opposite side of the film is created that is directly proportional to the change in bound, or absorbed, mass.
Microscale Thermophoresis (MST)
The technique of microscale thermophoresis allows for the detection of conformational changes in molecules that can be a consequence of binding, aggregation or polymerization, provided the change results in a change in mass, charge or solvation shell. This approach is well-suited for measuring equilibrium binding affinities. The techniques require the presence of a fluorophore and can be accomplished with small samples of analyte (8 uL). The DDRC hosts a NanoTemper MST monolith 115. 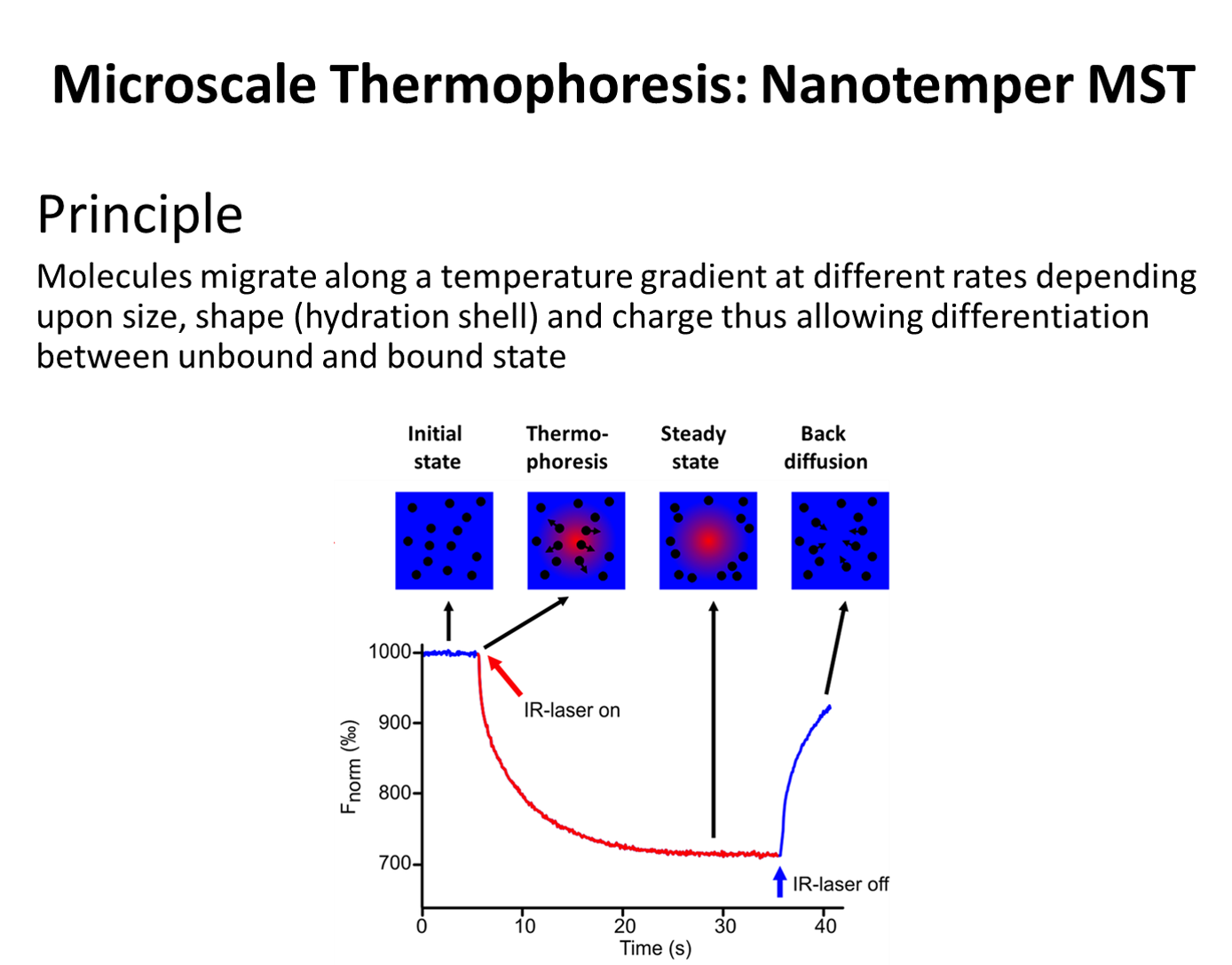
Temperature-Related Intensity Change (TRIC)
TRIC stands for Temperature-Related Intensity Change. Fluorescence is temperature- sensitive; fluorescence typically decreases with temperature, but the opposite is also possible. A change in fluorescence upon temperature increase is caused by collisions of the dye with neighboring molecules, resulting in radiation-free energy transmission. The temperature-sensitivity of fluorophores can be altered by a change in the environment of the fluorophore, such as an interaction of the target with a small molecule drug. The Dianthus instrument at DDRC is capable of performing high-throughput analyses of ligand receptor binding, or fragment-based screening, in a rapid, mix-and-read format.
Spectral Shift Analysis
Spectral shift analysis on the Dianthus instrument by NanoTemper is used to measure molecular interactions, such as binding between a protein and a ligand. One of the molecules is fluorescently labeled, and binding causes a subtle change in the local environment of the fluorophore. This results in a measurable shift in the fluorescence emission spectrum. The Dianthus detects these shifts with high sensitivity in a high-throughput, plate-based format. By analyzing how the spectral signal changes across ligand concentrations, binding affinity (Kd) can be accurately determined.
Circular Dichroism
Circular dichroism (CD) spectrometry measures very small differences in the absorption of right- and left-circularly polarized photons. This differential absorption correlates with the phenomenon of chirality. Changes in the CD spectrum correlate with protein secondary structure such as alpha-helixes or beta-sheets. Thus, CD spectrometry can be used to monitor changes in the secondary structure of biomolecules that possess chiral centers.
The DDRC has an Applied Photophysics Chriascan V-100 Circular Dichroism Spectrometer equipped with a heat ramp which allows us to monitor spectral changes during unfolding. We can provide guidance and training for its use, particularly in quality control studies or measuring sample variation due to ligand binding.
Thermal Melt Analysis
Thermal melt analysis (TMA) is a technique which can be used to measure the effect on stability of binding of small molecules to proteins. The instruments we support, are called the Bio-Rad CFX and the Nanotemper Prometheus Panta. These instruments generate a thermal ramp and measure the changes in fluorescence intensity of the sample, which contains a mixture of protein and ligand. A shift in the melting curve happens when the protein is bound to a ligand. The melting temperature dependence on ligand concentration can be fit to derive an affinity constant or Kd.
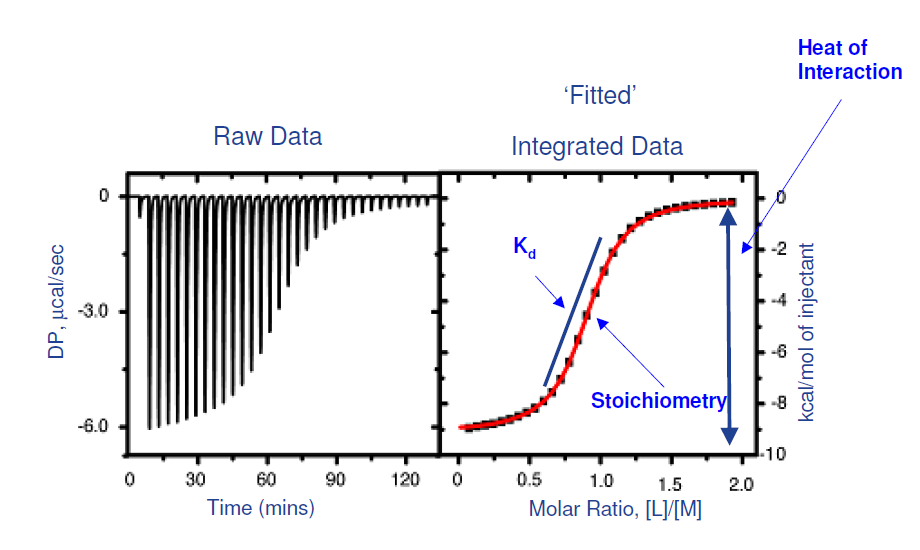
Isothermal Titration Calorimetry
The Malvern ITC PEAQ system directly measures heat released or absorbed during biochemical binding events, from which it calculates binding affinity (KD), stoichiometry (n), enthalpy (ΔH), and entropy (ΔS).