News and Announcements
Unveiling the Microscopic Universe: Contributions from Rockefeller’s Electron Microscopy Resource Center

The Rockefeller University’s Electron Microscopy Resource Center has co-authored significant findings on mitochondrial dynamics in a recent study published in Nature, advancing our understanding of cellular metabolism.
Since Keith Porter’s pioneering electron micrograph of a cell in 1945, The Rockefeller University’s Electron Microscopy Resource Center (EMRC) has been a cornerstone in the microscopic exploration of biological structures. Over the decades, the center has consistently pushed the boundaries of cellular visualization, enhancing our understanding of cell structure, protein dynamics, and the mechanisms of infectious diseases.
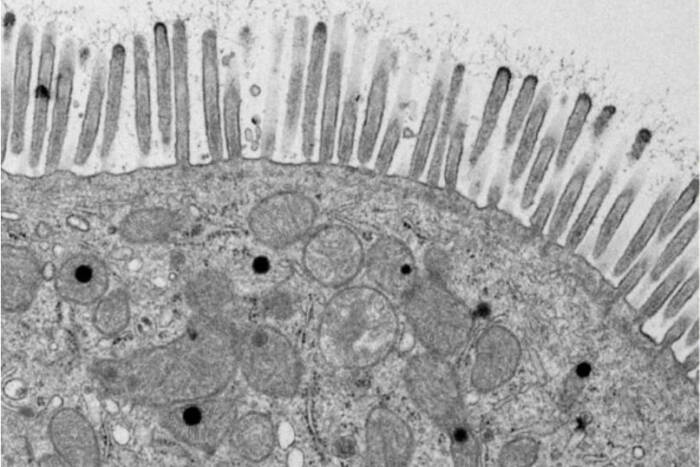
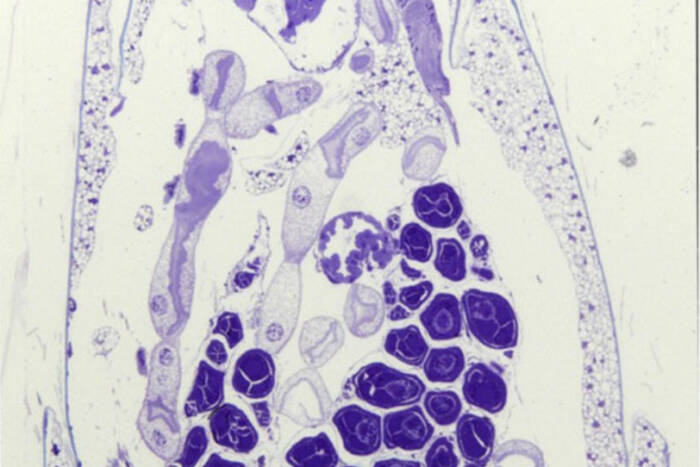
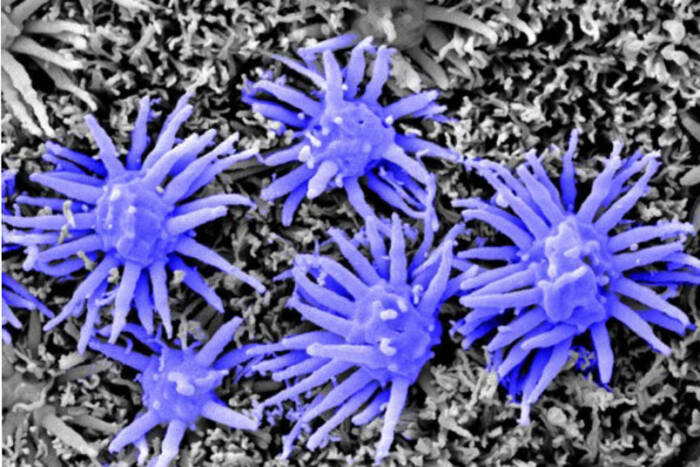
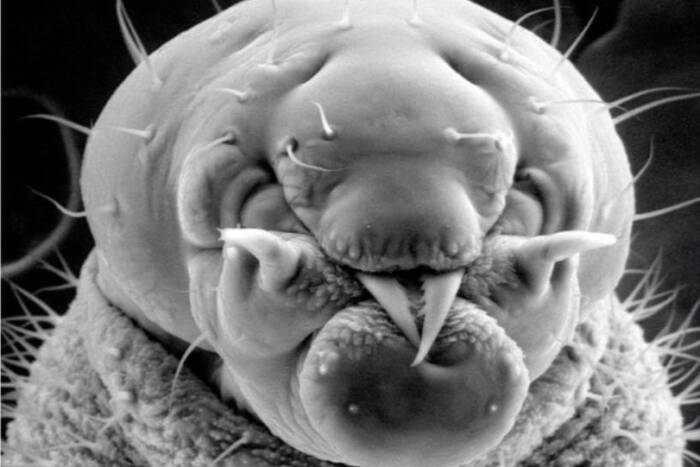
Today, the EMRC continues to lead with groundbreaking techniques in electron microscopy. From studying traditional specimens like bacteria and yeast to venturing into uncharted territories with ants and mosquitoes, the center is a hub for both innovation and tradition.
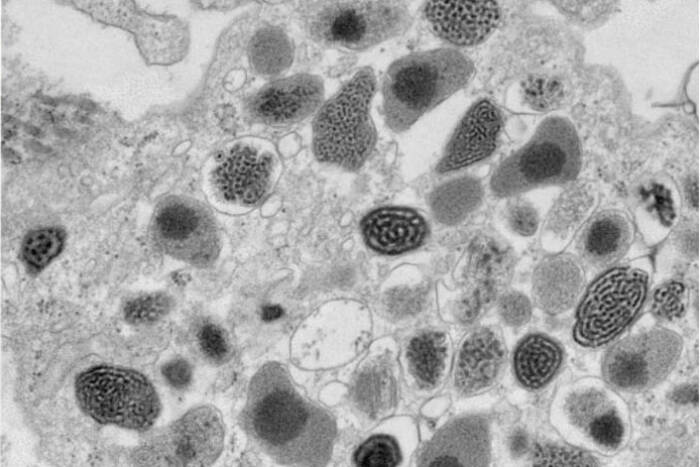
Dr. Amalia Pasolli, Director of the EMRC, and Dr. Anurag Sharma recently co-authored a study in Nature titled “Cellular ATP Demand Creates Metabolically Distinct Subpopulations of Mitochondria,” [1] which uncovers how cellular energy requirements influence mitochondrial dynamics and function. This research demonstrates the adaptive capabilities of mitochondria to segregate metabolic pathways in response to cellular energy demands. The findings, facilitated by the advanced imaging technologies at the EMRC, have profound implications for understanding metabolic disorders and cancer. The full article is available here(opens in new window)
“Developing new methods for difficult-to-fix samples is at the heart of our mission,” says Dr. Pasolli. “Techniques like Cryo-Scanning Electron Microscopy and High-Pressure Freezing allow us to preserve and examine specimens in their near-native states, opening up new realms of understanding.”
The Electron Microscopy Resource Center boasts a range of sophisticated techniques and tools that enable detailed studies of biological processes. Cryo-Scanning Electron Microscopy (Cryo-SEM) serves as a critical tool for exploring invertebrates, shedding light on the intricate details of their natural environments. Bridging the technological gap, Correlative Light and Electron Microscopy provides a seamless connection between conventional light microscopy and transmission electron microscopy (TEM), allowing researchers to achieve a comprehensive view of cellular activities. For those seeking dynamic insights, 3D TEM Tomography offers a cinematic glimpse into cellular functions, producing vivid “movies” of mitochondria in motion. Additionally, the center employs Immunogold Labeling to precisely target specific antigens within cells, significantly enhancing the detection and detailed study of essential cellular components.
“Our focus remains on supporting Rockefeller University projects, but our expertise is in high demand across the country,” explains Dr. Pasolli. The center’s proficiency in skin ultrastructure and collaborations, like those with Janelia Research Campus on Volume and 3D EM projects, underscore its pivotal role in the broader scientific community.
“Under Amalia’s leadership, the EMRC has not only honored its storied past but also moved forward in the field of electron microscopy,” remarks Mary Beth Hatton, Frederick P. Rose Professor and Head of Laboratory of Neurosciences and Behavior. “The EMRC’s support is essential to our ongoing study of neurodevelopmental disorders.”
For more information about the Electron Microscopy Resource Center and its projects, visit the Rockefeller University EMRC.
References:
[1] Ryu KW, Fung TS, Baker DC, et al. Cellular ATP demand creates metabolically distinct subpopulations of mitochondria. Nature. 2024 Nov 6; doi: 10.1038/s41586-024-08146-w. Online ahead of print. PMID: 39506109