Enzyme structure helps to explain how protein factories are constructed
A cell is more or less a sack of proteins. Ribosomes are cellular structures that produce those proteins. But how are ribosomes themselves produced?
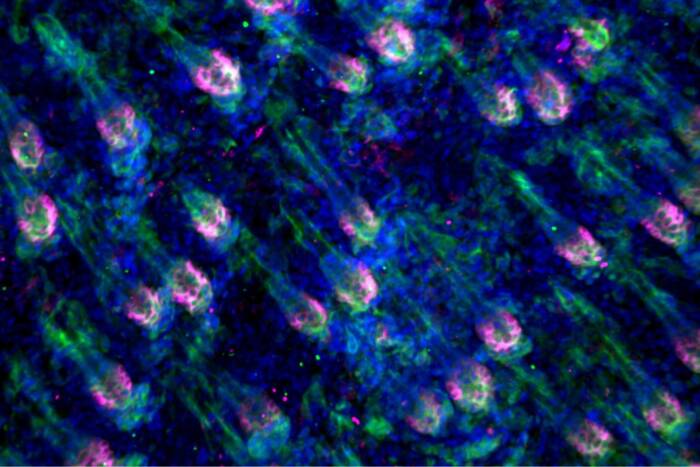
Rockefeller scientists Tarun M. Kapoor, Thomas Walz, Zhen Chen, and Hiroshi Suzuki recently made major strides toward answering this question. In a new study, published in Cell, the group describes the structure of Mdn1, an unusually large enzyme known to play a role in ribosome assembly.
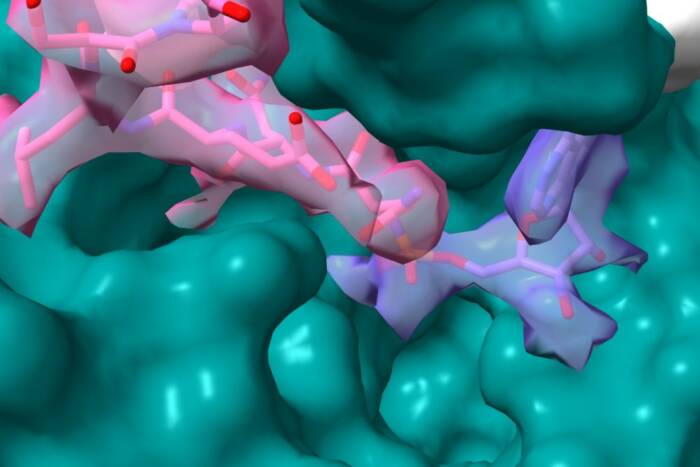
This research builds on a 2016 study, in which the same team discovered that the compound Rbin-1 targets and deactivates Mdn1—a finding that Kapoor, the Pels Family Professor, is now using to develop medication that blocks ribosome construction in pathogenic fungi.
In their latest study, the researchers again used Rbin-1 to target Mdn1; however, they did so not to deactivate the enzyme, but to freeze it in place, rendering it easier to analyze. Using this approach, the team characterized Mdn1’s structure, and learned that the enzyme can take one of two distinct shapes. This finding gives scientists a better grasp of how Mdn1 prompts precursor molecules to assemble into functional ribosomes.