Living cells prosper without telomeres
In most cells, telomeres are a critical protection against death: If these caps at the ends of chromosomes fail, the cell’s life is cut short. But what’s true for most cells isn’t true for all cells, and a surprising new finding from Rockefeller University, recently published in Genes and Development, shows that cells in the livers of living mice have the remarkable ability to prosper without telomeres.
 (opens in new window)
(opens in new window)
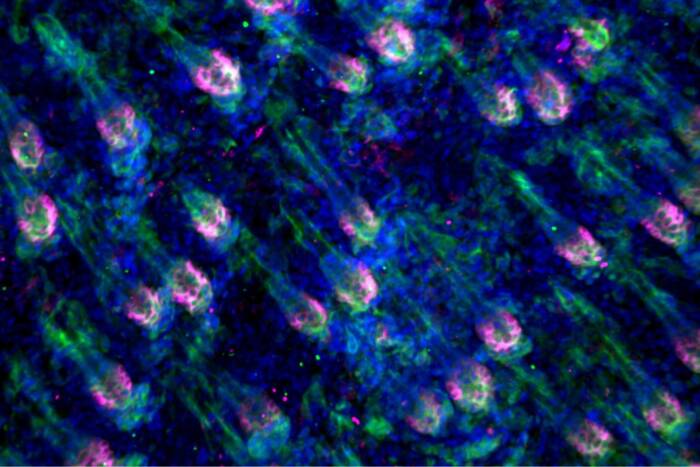
Life without telomeres. In cells from the livers of mice lacking a key telomere protein, TRF2 (bottom), chromosomes replicate and form long chains, but the cells don’t divide — causing the nuclei to swell to more than double their normal size.
Telomeres, the protein-DNA caps that protect the ends of chromosomes, are the focus of the research in Rockefeller’s Laboratory of Cell Biology and Genetics headed by Leon Hess Professor Titia de Lange. One of her lab’s pivotal discoveries is a protein complex called shelterin that protects telomeres. When telomeres lose shelterin, cells mistake the exposed chromosome ends for sites of DNA damage. The DNA damage signal elicited by dysfunctional telomeres usually kills cells. If cells try to repair the chromosome ends, they make such a mess of their chromosomes that they can no longer divide.
“But all our previous work on shelterin was done in tissue culture cells that are continuously dividing,” de Lange says. “We wanted to look at telomeres in cells that aren’t dividing — and nothing prepared us for the outcome.”
The researchers chose mouse liver cells as the subject because it’s easy to delete specific genes in the liver, and although liver cells do not normally divide, they will if the organ is injured.
When de Lange and her colleagues, Eros Lazzerini Denchi and Giulia Celli, deleted the essential shelterin protein TRF2, they anticipated telomere dysfunction and cell death — just as they had observed in cell culture. And, at first, everything happened as expected. There was a brief DNA damage response, and the telomeres were repaired inappropriately. But the cells did not die, “and Eros told me that the mice were fine,” de Lange says. Further examination of the cells revealed that all of the chromosomes were stuck together in long trains, yet gene expression was normal, producing all the enzymes the liver needs to dissolve fats, break down hormones and do its other metabolic work.
Even more surprising was the outcome when Lazzerini Denchi forced the regeneration of the livers by performing partial hepatectomy. In this well-established procedure, the remaining liver cells divide several times and regenerate liver mass in that way. Again, they found that absence of shelterin was not harmful and the mice regenerated their livers with no difficulty.
“To say we were surprised is an understatement,” de Lange says. “When shelterin was absent, the liver regeneration took a completely different route. Instead of making more cells by dividing, the remaining liver cells just got larger, with big nuclei that contained more DNA than normal liver cells, yet they were fully functional. It appears that liver cells are so flexible with their genomes that they have other ways of making up for severe damage.”
The study raises several questions, and de Lange is interested in understanding how liver cells know not to divide after they duplicate their chromosomes, but just get bigger. “This is a real puzzle and we would like to understand how it works,” de Lange says.