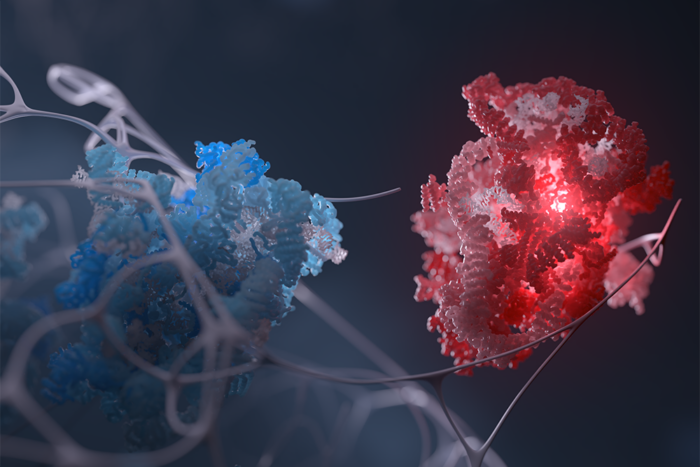
Protein structure suggests bacteria may be more sophisticated than we thought
When a bacterium or virus infects a plant, the plant fights back: It kills off its own cells in the area that’s infected. This immune response, known as programmed cell death, kills the invading organisms, limits its spread in the plant, and results in characteristic brown patches on the plant’s leaves. But some bacteria have evolved their own counterattack. Pseudomonas syringae, a plant pathogen that attacks a number of species, has developed a way around this slash-and-burn defense.
 (opens in new window)
(opens in new window)
A new leaf. Two leaves from the same tomato plant: Researchers injected the leaf on the left with three different versions of AvrPtoB — all of which had been mutated in a way that prevented ubiquitination, allowing the plant to kill off infected cells (green areas). The leaf on the right was injected with another mutant (top left), an empty control vector (bottom left), and intact AvrPtoB (right). The loss of its E3 ubiquitin ligase left the bacteria susceptible to the plant’s slash-and-burn defenses, resulting in fewer dead areas.
Just how it does it has remained a mystery, but researchers do know that one of the bacterium’s proteins, called AvrPtoB, plays a vital role in suppressing a plant’s immune response and preventing programmed cell death. To find out more about the function of AvrPtoB, scientists at Rockefeller University have imaged this molecule for the first time, and what they found amazed them: The protein appears to have the same structure and function as those that, until now, had been associated primarily with eukaryotic cells.
In an advanced online paper published today inScience Express, C. Erec Stebbins, assistant professor and head of Rockefeller’s Laboratory of Structural Microbiology, and Radmila Janjusevic, a postdoctoral associate in the lab, used x-ray crystallography to determine the structure of AvrPtoB. They found that the molecule is shaped like eukaryotic E3 ubiquitin ligases—enzymes responsible for attaching a small protein called ubiquitin to larger molecules, thereby marking them for destruction. “E3 ligases are very important,” Stebbins says. “They regulate cell cycle, apoptosis, development, and are deregulated in cancer. They’re absolutely central to what goes on in the cell.”
Stebbins and his collaborators at Cornell University found that not only is AvrPtoB the same shape as E3 ligases but that it also shares the ability to attach ubiquitin to molecules, leading the researchers to suggest that P. syringeae uses AvrPtoB to mimic this host activity to defeat plant defenses. To test this theory, they injected both original and mutated versions of AvrPtoB into the leaves of tomato plants, and compared their response. The leaves subjected to the intact bacterial protein showed little evidence of immune response; they remained green despite the infection, with no brown spots to indicate areas of programmed cell death. But leaves subjected to mutated AvrPtoB appeared to regain their ability to recognize bacterial invasion, and large brown patches appeared at the injection sites.
“This is exciting, because a bacterium has evolved what seems to be a host-like enzymatic activity, one that hasn’t ever been discovered in bacteria,” Stebbins says. “Historically, I think that bacteria have been viewed simplistically, as brutish invaders without very much sophistication. But reality couldn’t be further from this image. In some ways, bacteria are just as sophisticated — at times even more so — than viruses, or even the host cells that they both manipulate.”
Science Express: December 22, 2005(opens in new window)