For sex to happen, the right receptors must align
Having sex is largely about being in the right place at the right time. That’s true not only in the singles scene, but also at the molecular level. Research by Rockefeller’s Donald Pfaff, published this week in the online edition ofProceedings of the National Academy of Sciences, shows that the receptors for hormones that govern reproductive behavior must be in exactly the right place at the right time in order to interact.
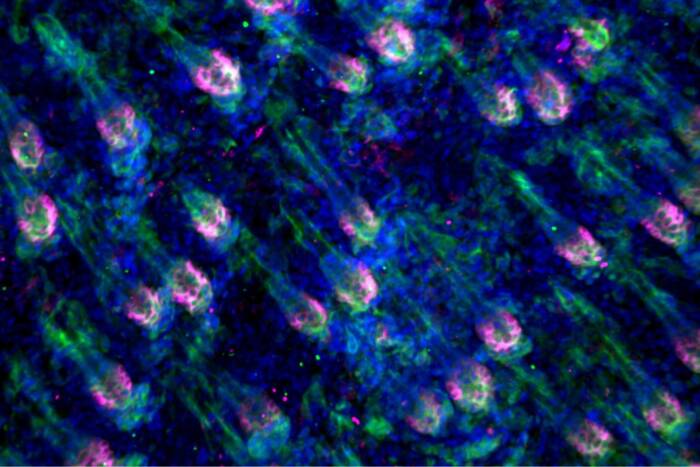
The neurons that are important for sexual behavior are located in an area of the brain called the ventromedial nucleus of the hypothalamus. These neurons, and the behaviors they govern, are highly responsive to the hormone estrogen, which signals through two receptor proteins called estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ). However, the data that have been collected have come from a pooled source of neurons. Pfaff and colleagues wanted to know how individual neurons were governed.
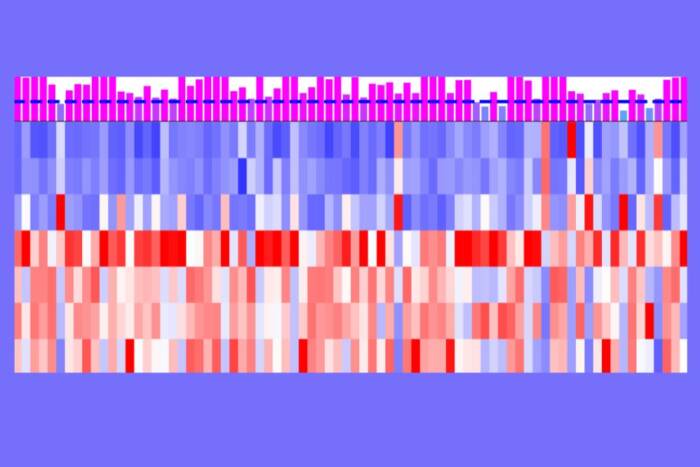
“We struggled at first,” says first author Nino Devidze, a postdoctoral fellow in Pfaff’s lab. “When you begin with a single neuron, you are starting with a very small amount of material. We tried many different methods, but finally found that real-time PCR gave us answers. We could take a single neuron from the ventromedial nucleus and ask what genes are expressed in that individual nerve cell.”
Real-time PCR, a technique that can amplify very small amounts of RNA, the blueprints for proteins, allowed the researchers to determine whether a specific set of genes were co-expressed in single neurons. Starting with ERα and ERβ, they looked to see if oxytocin receptors, also important for sexual behaviors, and a molecule called protein kinase C (PKC) were expressed in the same neuron. Then they compared results between male and female rats.
They found that even though ERα and ERβ are highly similar, they have different patterns of expression. Oxytocin receptors were almost always expressed in neurons that also had ERα, in both sexes. The surprise came when they looked at where different types of PKC, a protein that is important for delivering the estrogen and oxcytocin signals into the cell, were expressed. Males and females used different types of PKC to transmit signals from the estrogen and oxytocin receptors.
“Estrogen actions in female brains are well defined, but not so in males,” says Devidze. “Our data suggest that the theory of one gene equaling one behavior does not apply. Males and females both use estrogens and oxytocin to signal, with obvious differences in the resulting behaviors because the downstream pathways they activate, using specific types of PKC, are different.”
“Our results are a modest beginning to the discovery of the different genomic pathways involved in hormonal signaling,” Pfaff says. “They highlight the multiplicity and flexibility of signaling in the neurons of the hypothalamus. This work is a first attempt to piece together a signaling network in an individual type of neuron, and should encourage further searches to uncover additional genes involved in governing reproductive behaviors.”
PNAS 102(40):14446-14451 (2005)(opens in new window)