Natural-born killers enlisted to fight anthrax
Phage enzymes may offer powerful novel method to wipe out anthrax bacteria in seconds

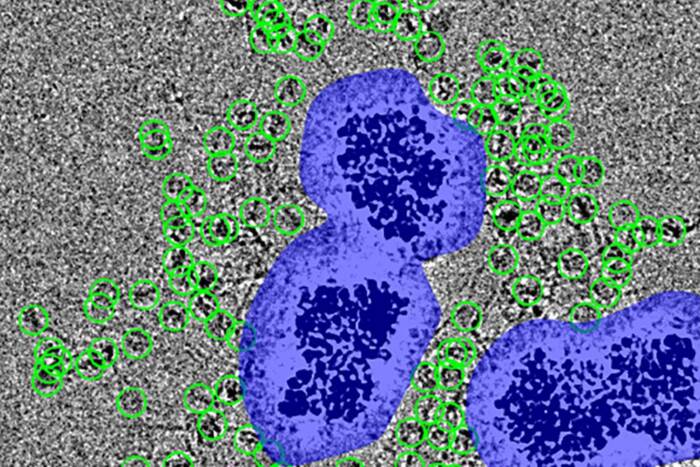
This series of electron micrographs shows the phage enzyme completely wiping out a colony of Bacillus cereus, the cousin strain of anthrax used in this study. The left panel shows a healthy colony of bacteria; the middle panel shows the bacteria 1 minute after enzyme treatment; and the right shows them 15 minutes after treatment.
Researchers at The Rockefeller University have hit upon a promising method for rapidly and effectively treating people infected with the deadly anthrax bacterium — including feared drug-resistant strains. The new research, reported in the August 22 issue ofNature, takes advantage of anthrax’s number one natural enemy: bacteriophage, or “bacteria-eating” viruses.
Bacteriophages, also known as phage, have been battling anthrax and other species of bacteria for billions of years. By isolating one of their primary weapons, the Rockefeller scientists have developed a powerful new agent that can specifically target and wipe out millions of anthrax bacteria within seconds. In addition, the technology shows promise as an anthrax detection and decontamination tool.
“We now know that anthrax is a real threat,” says Vincent A. Fischetti, Ph.D., head of the Laboratory of Bacterial Pathogenesis and Immunology at Rockefeller and principal author of the paper.
“But even more of a threat are multidrug-resistant strains of anthrax, which may occur naturally or may be engineered by terrorists using common molecular techniques. Consequently, alternative strategies for combating these dangerous strains are needed now more than ever.”
Funding this research is Defense Advanced Research Projects Agency (DARPA), the central research and development organization for the U.S. Department of Defense.
Phage (plural) are the most abundant life form on earth and can be found anywhere that bacteria thrive, such as soil, water and sewage. Like human viruses, they inject their genetic material into the bacterial cell, replicate by the hundreds per cell, then burst out before moving on to the next host cell.

Vincent Fischetti (left), Daniel Nelson and Raymond Schuch showed that 70 to 80 percent of mice infected with a less-infectious cousin strain of anthrax could be cured using the new phage enzyme.
In the Nature study, Fischetti and co-authors Raymond Schuch, Ph.D., and Daniel Nelson, Ph.D., focused on a phage that specifically infects the biowarfare agent Bacillus anthracis —anthrax. They isolated the enzyme that allows these phage to rupture and escape from the anthrax bacterium and showed that just drops of it can almost instantaneously destroy a test tube full of these deadly bugs—including the weaponized “Ames” strain, which was linked to the anthrax cases following September 11, 2001.
“This enzyme is almost as effective as pouring bleach over these organisms,” says Schuch, first author of the paper and a postdoctoral researcher at Rockefeller. “There is no other known biological agent that kills this quickly.”
But unlike bleach, this enzyme is what Fischetti calls a “targeted killer.” Because it is derived from a phage that specifically infects anthrax, it too will attack only this species.
“We tested the enzyme on many different types of bacteria, even related bacilli, such as the microbial insecticide Bacillus thuringiensis (also known as ‘Bt’), and found that it didn’t touch them,” says Fischetti. “This is beneficial because, unlike antibiotics, this kind of therapy would not kill off useful bacteria in our bodies and thus would have few or no side effects.”
The researchers also tested the enzyme on mice infected with a non-infectious close cousin strain of anthrax, called Bacillus cereus. All mice die within four hours of being infected with this pathogen. But when the researchers injected a dose of the new enzyme into the abdominal cavity of the mice 15 minutes after they were infected with B. cereus — also through injection into the abdominal cavity — 70 to 80 percent of them lived.
This similar strain of anthrax was chosen for study because it is easier to work with and therefore produced quicker results. Moreover, studies with this cousin strain could be performed in Fischetti’s laboratory at Rockefeller, while studies on the more dangerous strains of anthrax itself, including the “Ames” strain, were tested at the Centers for Disease Control and Prevention in Atlanta, Ga., and the Aberdeen Proving Ground in Aberdeen, Md.
Medicine and tool
In addition to treating anthrax infections, the new enzyme could potentially be used as both a decontaminant of anthrax-infected areas and as part of a hand-held anthrax detection device, says Fischetti.
These latter applications depend on the ability of this enzyme to detect anthrax spores. When anthrax is not busy infecting its human or animal hosts, it slips into a state of nearly permanent slumber, assuming the form of tough, infectious particles called spores. These spores can lie dormant for hundreds of years; only when they sense the presence of a new host will they wake up or “germinate” and start reproducing.
In the Nature report, the researchers show that this enzyme can be used in combination with a germinating agent to wipe out spores. The germinating agent, called L-alanine, tricks the anthrax into thinking it’s time to wake up. But before these sleepy bacteria have a chance realize they’ve been duped, the phage enzyme goes to work, killing them almost instantaneously.
Fischetti says that this lethal combination of enzyme and germinating agent could be used to both detect and eliminate spores that may lurk in mailrooms or subway stations, in addition to those stubborn few that might remain hidden in people’s noses.
Old strategy reinvented
The idea of using phage to fight bacterial infections is not new. Beginning in the 1930s, Soviet physicians employed whole phage, also referred to as “phage therapy,” to treat a variety of bacterial infections with moderate success. However, bacteria develop resistance to phage as they do to antibiotics, eventually rendering the treatment ineffective.
Fischetti’s new approach of using solely an enzyme derived from the phage gets around this obstacle. This particular enzyme, which is known as a lysin, helps newly assembled phage to escape from the bacteria by chewing up an essential cell wall bond, in effect punching “holes” in the bacteria’s outer barrier, which then causes it to explode. Thus, for the anthrax to develop resistance to the enzyme, it would have to alter this fundamental structure. According to the Rockefeller scientists, this would take a very long time.
“Phage have had a long time, perhaps billions of years even, to figure out what structures are vital to the bacteria,” says Schuch. “Consequently, it would take the bacteria more than a while to figure out how to fundamentally restructure their cell wall.”
The researchers demonstrated this advantage by showing that, under the same circumstances in which bacteria typically develop resistance to traditional antibiotics, not once did the cousin strain of anthrax develop resistance to the phage enzyme.
Universal approach
Fischetti first realized the medical potential of these enzymes about four years ago. Though he had used them as a tool to crack open bacteria in a test tube during his graduate school days, it wasn’t until the need for alternative strategies to antibiotics became increasingly pressing that he thought of using these enzymes to kill reservoirs of infectious bacteria in people. Since nearly all bacteria have bacteriophage systems, this strategy may be applied to most disease bacteria.
In February 2001, in the journal Proceedings of the National Academy of Sciences, he and his colleagues reported how a phage enzyme might be used to prevent strep throat and other infections caused byGroup A streptococcus, which normally reside in people’s throats. Using a spray containing a phage enzyme specific for this bacteria, they wiped out reservoirs of this infectious bug in the throats of mice (http://www.rockefeller.edu/pubinfo/pneumo121101.nr.php(opens in new window)).
In December of the same year, the researchers reported in Science the isolation of another phage enzyme, only this one was targeted at drug-resistant Streptococcus pneumoniae, the widespread infectious pathogen responsible for most cases of ear infections, pneumonia and bacterial meningitis. They again demonstrated the potential of the enzyme to prevent infections by using a nasal spray containing the enzyme to rid mice of populations of the bug behind the nose and throat (http://www.rockefeller.edu/pubinfo/fischetti032001.nr.htm(opens in new window)). Clinical trials to test both of these enzymes in humans are in the planning stages.
The new phage enzyme specific for anthrax is different in that it would be used to treat, not prevent, infections. At a time when drug-resistant infections as well as bioterrorism are on the rise, this novel strategy could potentially save many lives, says Fischetti.
Founded by John D. Rockefeller in 1901, The Rockefeller University was this nation’s first biomedical research university. Today it is internationally renowned for research and graduate education in the biomedical sciences, chemistry, bioinformatics and physics. A total of 21 scientists associated with the university have received the Nobel Prize in medicine and physiology or chemistry, 16 Rockefeller scientists have received Lasker Awards, have been named MacArthur Fellows and 11 have garnered the National Medal of Science. More than a third of the current faculty are elected members of the National Academy of Sciences.