Study identifies signals that make early stem cells
Stem cells work throughout our lives as a sort of handyman, repairing damaged tissues and renewing some normal ones, like the skin we shed. Scientists have come to understand much about how stem cells function when we are adults, but less is known about where these stem cells come from to begin with, as an embryo is developing.
Now, researchers at The Rockefeller University have identified a new mechanism by which cells are instructed during development to become stem cells. The results, published in Cell on January 14, help explain how communication between cells mediates this process, and may have implications for skin cancer treatments.
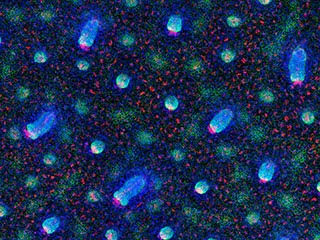
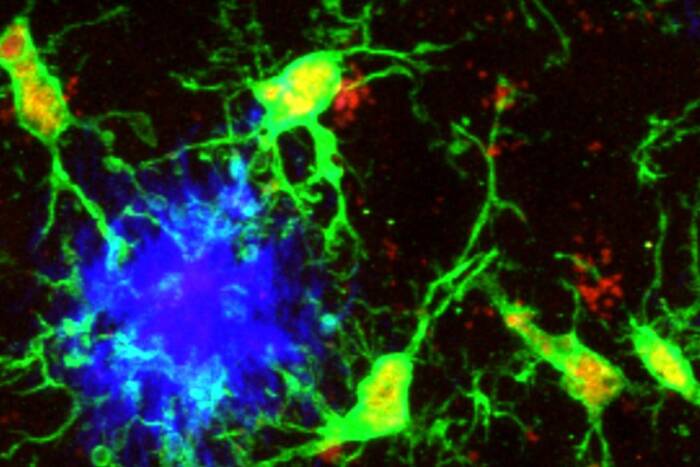
Stem cell debut: The researchers traced the cell divisions that occur as hair follicles form in mice to determine where stem cells first emerge. Above, developing hair follicles are shown at various stages.
“While adult stem cells are increasingly well-characterized, we know little about their origins. Here, we show that in the skin, stem cell progenitors of the hair follicle are specified as soon as the cells within the single-layered embryonic epidermis begin to divide downward to form an embryonic hair bud,” explains Elaine Fuchs, Rebecca C. Lancefield Professor and head of the Robin Chemers Neustein Laboratory of Mammalian Cell Biology and Development(opens in new window). “This timing was much earlier than previously thought, and gives us new insights into the establishment of these very special cells.”
Which came first, the stem cell or the niche?
Clusters of stem cells receive signals from other nearby cells that instruct them to either stay a stem cell or differentiate into a specific cell type. These instructive groups of cells, called the “niche,” are known to maintain adult stem cell populations. Less well understood is how the niche forms, or when and where stem cells first appear during embryonic development.
“Adult stem cells are dependent on the niche for instructions on both how to become a stem cell, and how to control stem cell population size,” says first author Tamara Ouspenskaia. “The question was, does the niche appear first and call other cells over to become stem cells? Or is it the other way around? Stem cells could be appearing elsewhere first and then recruiting the niche.”
Working in the mouse hair follicle, a region that contains active stem cells, Fuchs and colleagues investigated the cell divisions that occur as a hair follicle is first forming. The hair follicle begins as a small bud called a placode, and develops into a tissue of multiple layers, comprised of different cell types. By labeling cells within the placode and tracing their progeny, the researchers determined that from each division, one daughter cell stayed put, while the other escaped to a different layer.
Further experiments revealed that this escapee becomes a stem cell. This finding is significant, as it’s the earliest point in development that stem cells have been detected in this system, and it indicates that stem cells may exist before the niche is formed.
Flying the nest to become a stem cell
How cells become the cell type they’re destined to be—a liver cell or skin cell, for example—depends on a number of factors, including molecular signals from other cells that help turn specific genes on or off. Fuchs and colleagues observed that the signaling activity was different between the two daughter cells that ended up in different locations, and aimed to characterize how signaling helped seal their ultimate cell fate.
They found that the environment to which the escapee cell daughter moved had low levels of WNT signaling, known to play a role in embryonic development. In contrast, WNT signaling was high in the environment where the other daughter remained. The level of WNT affected how the cells responded to another signal known as SHH (Sonic Hedgehog)—only those in the low-WNT environment responded to SHH signaling, which instructed the cells to become stem cells.
“These cells must leave home, they must leave the environment with high WNT signaling, to become stem cells,” says Ouspenskaia. “We observed that SHH, which actually comes from the cells with high WNT signaling, is essential in helping the cells leave. So in order for this escapee cell to become a stem cell, it needs to receive an SHH signal from its sister cell at home telling it ‘you’re the stem cell.’”
The researchers believe that antagonism between WNT and SHH signaling may help to control the number of stem cells produced during this time of embryo development.
“This newly identified signaling cross talk provides insights into why these two signals have such a profound impact on skin cancers, where the numbers of cancerous tissue-propagating stem cells are excessive,” says Fuchs, who is also a Howard Hughes Medical Institute investigator. “This work now paves the way for future research into the fascinating and clinically important relation between tumor-propagating and normal stem cells.”
 (opens in new window)Cell 164, 156–169 (opens in new window)Cell 164, 156–169WNT-SHH Antagonism Specifies and Expands Stem Cells prior to Niche Formation(opens in new window) Tamara Ouspenskaia, Irina Matos, Aaron F. Mertz, Vincent F. Fiore, and Elaine Fuchs |