Scientists learn how young brains form lifelong memories by studying worms’ food choices
Members of neuroscientist Cori Bargmann’s lab spend quite a bit of their time watching worms move around. These tiny creatures, Caenorhabditis elegans, feed on soil bacteria, and their very lives depend on their ability to distinguish toxic microbes from nutritious ones. In a recent study, Bargmann and her colleagues have shown that worms in their first larval stage can learn what harmful bacterial strains smell like, and form aversions to those smells that last into adulthood.
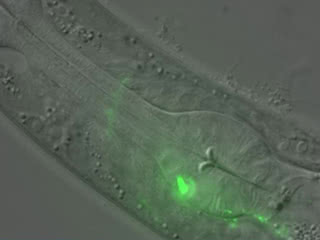
Lasting impressions: A segment of a C. elegans worm with a RIM neuron shown in green. The scientists showed that this neuron produces a learning signal that makes the newly hatched worm able to remember an olfactory experience for the rest of its life.
Many animals are capable of making vital, lifelong memories during a critical period soon after birth. The phenomenon, known as imprinting, allows newly hatched geese to bond with their moms, and makes it possible for salmon to return to their native stream after spawning. And while the learning processes of humans may be more complex and subtle, scientists have long known that our brain’s ability to store a memory and maintain it long-term depends on when and how that memory was acquired.
“In the case of worms, we were fascinated to discover that their small and simple nervous system is capable of not only remembering things, but of forming long-term memories,” says Bargmann, who is Torsten N. Wiesel Professor and head of the Lulu and Anthony Wang Laboratory of Neural Circuits and Behavior(opens in new window), as well as co-director of the new Kavli Neural Systems Institute(opens in new window) at Rockefeller. “It invites the question of whether learning processes that happen during different life stages are biologically different.”
In the study, she and Rockefeller graduate student Xin Jin let both young and adult worms learn to avoid food smells, and studied in detail the neural circuits that produced memories of the experience. Their findings, published today in Cell(opens in new window), clarify which neurons, genes, and molecular pathways distinguish the two types of memory, providing new vistas into the neurobiology of learning.
Imprinted aversions last a lifetime
When adult C. elegans worms encounter pathogenic bacteria, they avoid it by moving in the opposite direction, and they shun similar bacteria for about 24 hours. But their memory soon fades.
Young worms, on the other hand, form more lasting impressions. The researchers allowed newborn worms to hatch directly onto a lawn of pathogens, and left them there for their first 12 hours of life—the first larval stage. (The bugs gave the worms intestinal infections, but didn’t kill them.) Then, when the worms encountered the pathogens again as adults—three days later—they fled. Worms that hadn’t been hatched onto poisonous bacteria found them just as attractive as harmless ones.
Jin found that by silencing specific neurons in the worms, and repeating the learning assays, she was able to determine each nerve cell’s contribution to the memory process. The results of her experiments show that the neural circuits that mediate the two types of learning are similar, but not identical. Many neurons are needed for both imprinted and adult learning, but cells called AIB and RIM are uniquely important for the formation of the imprinted memory during the larval stage.
A similar picture emerged when the researchers compared the genes and signaling pathways that are activated when worms form imprinted versus short-term memories. The two processes rely on similar molecular components, but some genes were found to be specifically required for only one type of learning.
“These findings suggest that early imprinting isn’t totally different from other learning—it’s the same system enhanced with some special features,” Bargmann says.
How memories are formed, stored, and retrieved
Several neurological processes are at play when we learn new things. For example, when a baby songbird learns a song from an adult bird, a memory of the tutor’s performance must first form and be stored in his brain. Then, when it’s time for the bird to debut with his own song, that memory must be retrieved to practice and then perform a vocal behavior.
Because most animals’ brains are very complex, it has been difficult for scientists to study these elements of learning in detail. By using C. elegans, whose modest brain has only 302 neurons, the researchers were able to shed light on the neural circuits that drive the formation and retrieval of a worm’s memory—and the two processes turned out to be neurologically distinct.
“We learned that when worms form an early memory of a food smell, they use one set of neurons to plant that memory,” Bargmann says, “and later in life, when they encounter the same smell again, they use a different set of neurons to pull the memory out.”
How memories are stored in the brain—in what neurons they reside and what constitutes them at the molecular level—remains elusive. But Bargmann says her lab’s findings have laid the groundwork for future research into stored memory and other open questions.
“The most evocative thing about this work is that it reminds us that learning isn’t some fancy innovation of a complex brain,” she says. “It’s a fundamental function that any nervous system can perform.”
 (opens in new window)Cell 164, 632–643 (opens in new window)Cell 164, 632–643Distinct Circuits for the Formation and Retrieval of an Imprinted Olfactory Memory(opens in new window) Xin Jin, Navin Pokala, and Cornelia I. Bargmann |


