New insights into muscular dystrophy point to potential treatment avenues
The average healthy man is 54 percent muscle by mass, but people with muscular dystrophy, an incurable, genetic condition, have almost no muscle at terminal stages of the disease. New research from The Rockefeller University provides insights about what causes patients’ muscles to degenerate and offers potential avenues for drug development.
In work published recently in Nature Communications, the team led by professor Sidney Strickland identified a group of proteins whose function appears to prompt the production of muscle.
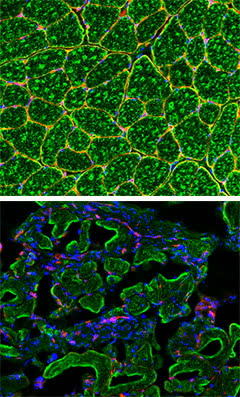
A muscular deficit: When the researchers shut off the expression of laminin (red) in pericyte and PIC stem cells, the affected mice (bottom) showed a dramatic alteration in muscle fiber maturation (green), as is seen in muscular dystrophy patients. Normal mouse muscle appears on top.
“Certain rare stem cells have the potential to turn into either fat or muscle, and we found that the decision hinges on a protein complex known as laminin,” says first author Yao Yao, a former postdoc in Strickland’s Patricia and John Rosenwald Laboratory of Neurobiology and Genetics and now an assistant professor at the University of Minnesota. “Our experiments in mice suggest that it may be possible to develop new drugs that, by acting on the laminin pathways, could relieve many of the symptoms of muscular dystrophy.”
A muscular fate
Muscles are made up of millions of tightly packed cells. Even in adults, these cells are constantly regenerating to repair damage that may be due to anything from exercise to injury. While most new muscle cells are derived from a type of stem cell that only produces muscle, a fraction arises from pericytes and PICs, rare groups of stem cells that can become either muscle or fat.
The processes that dictate whether these stem cells differentiate into muscle or fat have been implicated in muscular dystrophy, but their molecular underpinnings have been largely unknown.
Using mouse models, researchers have previously shown that loss of laminin throughout the body causes at least some forms of muscular dystrophy. Yao and his colleagues focused on the role of laminin specifically in pericytes and PICs.
The team generated a strain of mice that lacked laminin in these stem cells. “These mice were significantly smaller than their littermates and had severe muscle deficits,” says Yao. “Pericytes and PICs represent a small fraction of muscle stem cells, so we were very surprised to see such dramatic symptoms of muscular dystrophy.”
A genetic switch
Yao and Strickland reasoned that replacing the laminin in these tissues might help these animals recover. And indeed, when the research team injected laminin directly into the mice’s muscle they found that they regained both muscle tissue and strength.
“In a mouse, we are able to effectively treat muscular dystrophy with laminin injections, but this could be difficult in humans,” says Yao. Laminin is made up of three subunits that together are too large to diffuse well through tissue. “Patients would require hundreds of injections. It simply isn’t feasible,” Yao adds.
Instead, the scientists focused their attention on understanding how laminin drives pericytes and PICs to become muscle. The research team found that laminin affects which genes are turned on and off in these cells.
One altered gene in particular stood out. “This gene, called gpihbp1, is found primarily in capillaries where pericytes reside, and previous research has shown that it is involved in fat metabolism,” says Yao. When laminin is deleted, gpihbp1 is no longer turned on in pericytes and PICs.
The researchers hypothesized that restoring gpihbp1 might drive the stem cells to become muscle cells rather than fat. They forced the pericytes and PICs lacking laminin to turn on gpihbp1 and found that these cells preferentially differentiate into muscle cells.
The research team is now searching for drugs that can increase gpihbp1 levels in pericytes and PICs, in hopes of resolving many of the symptoms of this devastating disease.
“Our data suggests that gpihbp1 could be a novel target for the treatment of muscular dystrophy,” explains Strickland, who is also vice president for educational affairs and dean of graduate and postgraduate studies.
This work was supported by the National Institutes of Health under grant NS050537, and with a Myotonic Dystrophy Foundation Fund-A-Fellow Grant, Merck Postdoctoral Fellowship, and BD Stem Cell Grant.
 Nature Communications, online: May 3, 2016 Nature Communications, online: May 3, 2016Laminin regulates PDGFRβ+ cell stemness and muscle development Yao Yao, Erin H. Norris, Christopher E. Mason, and Sidney Strickland |