New mouse models give a boost to the development of cancer immunotherapies
Cancer immunotherapies—drugs that work by making a patient’s immune system better at spotting and destroying tumor cells—are increasingly generating headlines. A number of these drugs are now being used for the treatment of melanoma, lung, and kidney cancers, and are showing promise in clinical trials with other diseases as well.
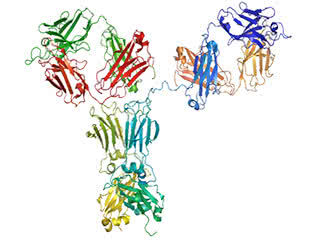
The immune trigger: Antibodies are Y shaped, and their stem, known as the Fc domain, can activate immune cells by binding to specific receptors on their surface.
But creating drugs that manipulate the immune system presents unique challenges. With several experimental treatments, initial studies done in cells and animals produced encouraging results, yet the drugs didn’t work as hoped in clinical trials. Among these ambiguous immunotherapies is a group of antibody drugs that target CD40, a protein present on certain immune cells that function to activate them.
“Antibodies that activate CD40 showed great promise in animal models at Rockefeller and elsewhere, but in several international patient trials we’ve seen a big gap between that promise and clinical efficacy,” says Rony Dahan, a postdoctoral fellow in Rockefeller University’s Laboratory of Molecular Genetics and Immunology, led by Jeffrey Ravetch.
Now, Dahan and his colleagues have created a new mouse model that allowed them to evaluate CD40-antibody drugs with improved accuracy, and advance those that are more likely to be effective in patients. Results from their experiments in these mice are reported in Cancer Cell.
Building a better mouse
Drugs that activate immune cells by targeting CD40 proteins on their surface have been shown to promote antitumor responses in several ways, and each response is initiated by immune cells called dendritic cells. Scientists consider these compounds to be promising because they have been shown to elicit more specific immune responses to tumors than other immunotherapies, and could be used in combination with other drugs.
But so far attempts to develop antibodies targeting CD40 have been disappointing. To find out why, the Rockefeller team engineered mice whose immune systems more closely mimic those of people. The new mouse model expresses the human versions of both the CD40 protein and of Fc receptors, a group of proteins expressed on immune cells. The Fc receptors bind to the back side of antibody molecules, in a region known as the Fc domain.
Once the researchers were able to develop receptors that are more like those seen in patients, they were able to look for antibodies that bind them more tightly. Different Fc receptors are expressed on different types of immune cells and carry different immunologic properties. The researchers discovered that engagement of a certain human Fc receptor, called FcRIIB, is essential for the therapeutic activity of human CD40 antibodies. However, engagement of a different receptor, FcRIIA, compromises their activity.
These findings indicate that when it comes to human CD40 antibodies, the Fc domain is important for inducing an effective immune response against tumors.
“We know from our study that current antibodies under development don’t fully utilize the potential of the CD40 approach,” Dahan says. “We have used our new model to identify and select new Fc-engineered CD40 antibodies that have significantly enhanced antitumor activity. We then advanced the most promising candidate into clinical trials of various solid tumor types, in a project funded by Rockefeller’s Robertson Therapeutic Development Fund.”
This research was supported by the National Institutes of Health (grant HHSN261200800001E) and the Robertson Therapeutic Development Fund.
 Cell, online: June 2, 2016 Cell, online: June 2, 2016Therapeutic Activity of Agonistic, Human Anti-CD40 Monoclonal Antibodies Requires Selective FcγR Engagement Rony Dahan, Bryan C. Barnhart, Fubin Li, Aaron P. Yamniuk, Alan J. Korman, Jeffrey V. Ravetch |