Study explains how an intestinal microbe protects against other, more dangerous bacteria
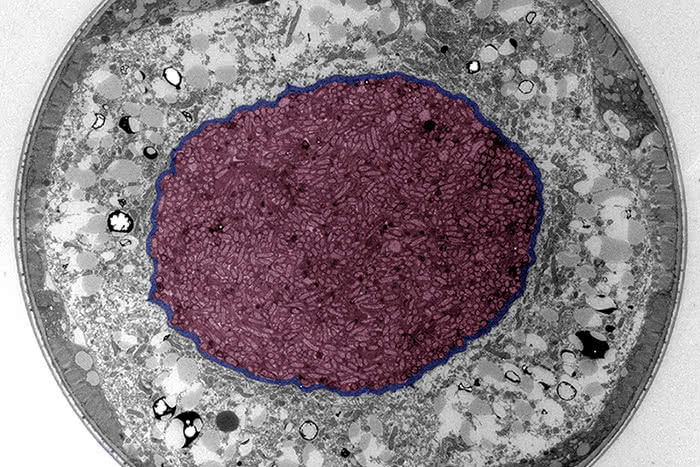
Bad stomach: The researchers performed experiments in C. elegans worms infected with Salmonella bacteria. In this cross section of a worm, the rod-shaped microbes have invaded the animal’s intestine, highlighted in purple.
Antibiotics save millions of lives. But their tendency to kill helpful and harmful bacteria alike, coupled with the growing problem of antibiotic resistance, means that they are not without their downside.
Probiotics consisting of beneficial microorganisms, meanwhile, have the potential to deliver the benefits of antibiotics minus the pitfalls. Yet up until now, evidence of their efficacy has been largely anecdotal, their mechanisms of action poorly understood.
Thanks to a pair of papers recently published in Science and Science Immunology by researchers at The Rockefeller University, however, that is beginning to change.
The studies demonstrate that an enzyme produced by a common intestinal microbe can protect the guts of worms and mammals alike from attack by harmful bacteria, and offer important insights into how it does so. Together, their findings could lead to the development of probiotics for use against such dangerous pathogens as Clostridium difficile, a leading cause of hospital-acquired infections.
A bug with therapeutic potential
The researchers set out to investigate the probiotic potential of the microbe Enterococcus faecium in the roundworm Caenorhabditis elegans. Although E. faecium has long been used as a probiotic in livestock, its mode of action has never been clear. And it is far from being an ideal probiotic for use in humans: according to Kavita Rangan, first author of the Science paper and a member of Howard Hang’s Laboratory of Chemical Biology and Microbial Pathogenesis, E. faecium readily acquires antibiotic resistance in hospital settings and can lead to dangerous infections in people with compromised immune systems.
Yet in a series of experiments, Rangan and her colleagues demonstrated that when fed E. faecium, C. elegans was better able to resist the harmful effects of infection by Salmonella typhimurium, an intestinal pathogen that in mammals invades the thin layer of epithelial cells lining the gut. “Salmonella was still able to colonize the intestine,” says Rangan, “but it didn’t cause the same tissue damage to the worms, and it didn’t kill them.”
What’s more, they discovered that a particular enzyme called SagA, which is secreted in abundance by E. faecium, was sufficient to protect both worms and mice from Salmonella. And they showed that SagA worked its magic in mice even when produced by a different microbe called Lactobacillus plantarum—an entirely innocuous bug that is commonly used as a probiotic for human intestinal diseases, and which naturally inhabits environments ranging from sauerkraut to the human gut.
Making good bacteria better
In a series of complementary experiments, Virginia Pedicord—first author of the Science Immunology paper, and a postdoctoral fellow in both the Hang lab and Daniel Mucida’s Laboratory of Mucosal Immunology—and colleagues also showed that E. faecium protected mice against S. typhimurium. In addition, they demonstrated that E. faecium prevented the pathogen from passing through the epithelium and invading other organs such as the liver.
Those experiments proved that E. faecium did not protect the mice by attacking S. typhimurium directly or by changing the balance of other microbes in the gut. “It doesn’t kill the bacteria, and it doesn’t deplete the microbiota, either,” Pedicord says. “It just prevents them from causing disease.” And it accomplishes this, she explains, by stimulating the production of specialized proteins that prevent pathogens from coming into contact with the epithelial layer in the first place—proteins that are generated by the epithelial cells themselves.
The team confirmed that, as was the case with C. elegans, SagA was by itself sufficient to protect the mice from the ravages of S. typhimurium. And it also identified a clutch of receptors and antimicrobial peptides related to the innate immune system that must be present for the enzyme to do its work.
But perhaps most strikingly, Pedicord and her colleagues showed that, when delivered by L. plantarum, SagA also protected the mice against C. difficile, a pathogen that causes debilitating and sometimes fatal gastroenteritis in human beings.
C. difficile sickens nearly 500,000 people in the United States each year and kills more than 29,000. Long-term antibiotic therapy for other conditions actually heightens the risk of infection, and treatment of C. difficile with antibiotics often leads to relapse.
As a result, the prospect of a benign probiotic that could defend against C. difficile while avoiding the problems associated with antibiotic treatment is welcome news.
“This is something that might really help people,” says Pedicord, who is already conducting experiments in mice to see if SagA has an effect on C. difficile recurrence.
This work was supported by grants from the NIH–National Institute of General Medical Sciences grant (R01GM103593), the Robertson Therapeutic Development Fund, the Lerner Trust, the Leona M. and Harry B. Helmsley Charitable Trust, and other foundations.
 Science 23 Sep 2016, Vol. 353, Issue 6306, pp. 1434-1437 Science 23 Sep 2016, Vol. 353, Issue 6306, pp. 1434-1437A secreted bacterial peptidoglycan hydrolase enhances tolerance to enteric pathogens Kavita J. Rangan, Virginia A. Pedicord, Yen-Chih Wang, Byungchul Kim, Yun Lu, Shai Shaham, Daniel Mucida, Howard C. Hang |
 Science Immunology 22 Sep 2016, Vol. 1, Issue 3, pp. eaai7732 Science Immunology 22 Sep 2016, Vol. 1, Issue 3, pp. eaai7732Exploiting a host-commensal interaction to promote intestinal barrier function and enteric pathogen tolerance Virginia A. Pedicord1, Ainsley A. K. Lockhart, Kavita J. Rangan, Jeffrey W. Craig, Jakob Loschko, Aneta Rogoz, Howard C. Hang, and Daniel Mucida |