A possible explanation for why male mice tolerate stress better than females
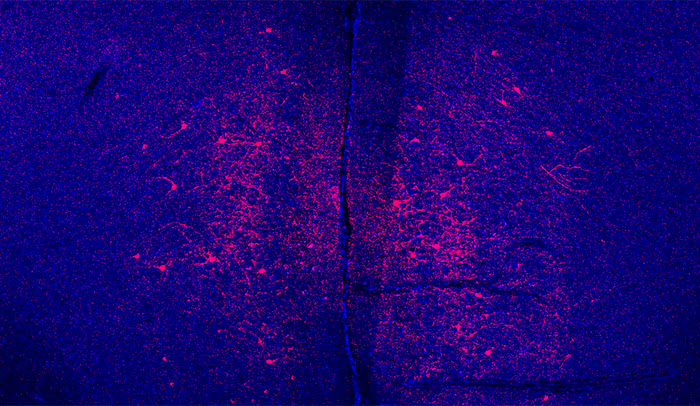
Same-same, but different: When the researchers activated certain brain neurons, shown in red, the effects were different in male and female mice: males became less anxious, while females became more social.
The nerves we feel before a stressful event—like speaking in public, for example—are normally kept in check by a complex system of circuits in our brain. Now, scientists at Rockefeller University have identified a key molecule within this circuitry that is responsible for relieving anxiety. Intriguingly, it doesn’t appear to reduce anxiety in female mice, only in males.
“This is unusual, because the particular cell type involved here is the same in the male and female brain—same in number, same in appearance,” says Nathaniel Heintz, head of the Laboratory of Molecular Biology and a Howard Hughes Medical Institute investigator. “It’s a rare case where a single cell type is activated by the same stimulus but yields two different behaviors in each gender.”
Heintz and colleagues demonstrated that a protein called corticotropin releasing hormone binding protein (CRHBP) reduces anxiety in male mice by halting the activity of a stress-inducing hormone. Published in Cell, the results may provide insights into new therapies for anxiety-related conditions.
Divergent effects
It’s a well-known fact that our social and emotional behaviors—and disorders associated with these behaviors— vary between men and women. For example, autism is more prevalent among men, while anxiety-related disorders tend to be more common in females. Differences in hormone levels and brain circuitry are thought to contribute to this variation, but the specific mechanisms responsible are not well understood.
Previous work in the Heintz lab, however, has provided one possible explanation. The researchers characterized a novel population of neurons that are activated by oxytocin, a hormone that promotes social behaviors like bonding between mother and baby or teamwork. They found that these neurons fire more rapidly in response to oxytocin in female mice, and are important for promoting certain social behaviors in which females interact with males, but had little affect on male social behavior.
In this study, the researchers asked what this particular cell type does in a male brain, suspecting from previous research on oxytocin that the stress response may be affected. Using optogenetics, a technique in which these neurons were engineered to fire in response to light, they examined the mice performing several tasks to test their anxiety levels.
“If mice are anxious, they won’t go out into unprotected areas,” says Heintz. “We found that if you activate these cells in males, they will leave the protected area more often, meaning they are less anxious. But in females, activating these cells made no difference in anxiety.”
These findings suggest that different behaviors are affected in male and female mice when these neurons are activated by oxytocin: anxiety is reduced in males, while social behavior is increased in females.
Same circuit, different sensitivities
Heintz and colleagues next sought to identify the molecular mechanisms at play in these varied responses. Using a technique known as TRAP—previously developed by the Heintz lab and Paul Greengard’s Laboratory of Molecular and Cellular Neuroscience at Rockefeller—they looked for proteins produced by the oxytocin-sensing neurons. The CRHBP protein was among the most abundantly produced in these cells.
“Our findings led us to propose a signaling pathway in these neurons in which oxytocin stimulates production of CRHBP,” says Heintz. “CRHBP then binds to a hormone called corticotropin-releasing hormone, preventing it from performing its normal job to increase stress.”
The question then became, why is the anxiety-reducing effect of CRHBP dominant in males, but doesn’t seem to work in females? Further experiments revealed that corticotropin-releasing hormone levels are much higher in females to begin with. One possibility, Heintz says, is that CRHBP can’t lower corticotropin-releasing hormone levels enough in females to make a difference. In males, however, it lowers the level of the stress hormone below a threshold that matters behaviorally, and effectively decreases stress and anxiety.
These results may explain how oxytocin can both promote social behaviors in females and alleviate stress and anxiety in males. And they suggest that while the brain circuits that control male and female behaviors may look exactly the same, they still function differently because they don’t respond the same way to certain hormones.
“But even though our findings may provide some insight into gender differences, they are even more important for understanding what may be different between individuals,” Heintz says. “Emotional and social behaviors are complicated, so finding any clues to why some people are more vulnerable to anxiety than others, or why some are social while others aren’t, matters. These are fundamental questions of human behavior that we don’t yet understand fully.”
This work was supported by the Howard Hughes Medical Institute, the National Institutes of Health/National Institute on Drug Abuse (grant 1P30 DA035756-01), the Leon Black Family Foundation, and the Human Frontier Science Program postdoctoral fellowship (LT000271/2015-L).
 Cell 167, 60-72 Cell 167, 60-72A Cortical Circuit for Sexually Dimorphic Oxytocin-Dependent Anxiety Behaviors Kun Li, Miho Nakajima, Ines Ibañez-Tallon, and Nathaniel Heintz |