Immune system uses a “leash” to restrict HIV’s spread
Measure. Countermeasure. So the war against a virus unfolds. The immune system comes up with one defense and the invader finds a way to thwart it. New research on HIV details a new example of this ongoing struggle, showing that the immune system produces an antiviral protein called tetherin that literally lashes viruses to the surface of infected cells, keeping the infection from spreading. Unfortunately, HIV has engineered a different protein to free itself.
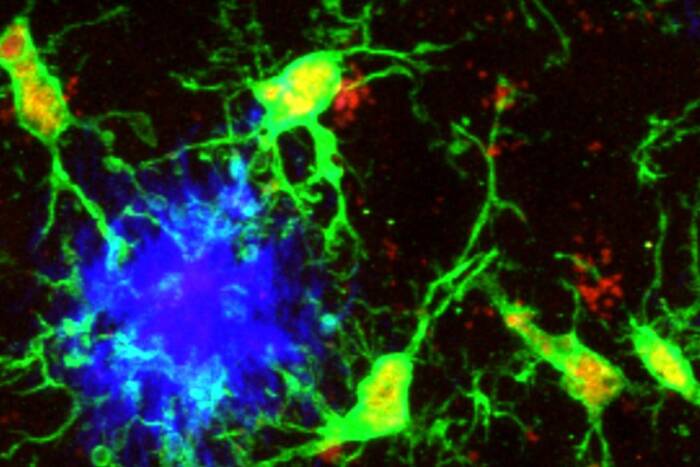
In experiments published October 29 in Cell, Rockefeller University’s Paul Bieniasz and colleagues in the Laboratory of Retrovirology(opens in new window) showed that tetherin, a “restriction factor” protein identified by the lab two years ago(opens in new window), is shaped like a rod with membrane anchors at each end. One anchor takes hold of the lipid envelope that houses HIV as newborn viral particles bud through the membrane of an infected cell. The other anchor remains embedded in the infected cell’s membrane, tying the virus in place and stalling further colonization. Led by research scientist David Perez-Caballero, the scientists demonstrated that tetherin requires both anchors for the leash to be effective.
 (opens in new window)
(opens in new window)
Lashing HIV. Without tetherin to stop it, HIV buds unencumbered through the membrane of an infected cell (top). With the protein (bottom), viral particles are leashed to the cell membrane and are unable to escape to infect other cells.
Based on the predicted rod-and-anchor structure of tetherin, researchers then designed an artificial protein with an amino acid sequence completely different from that of tetherin. They found that it performed the same function, strongly suggesting that tetherin’s structure and not the amino acid sequence is key, says Bieniasz, who is also a Howard Hughes Medical Institute investigator at the Aaron Diamond AIDS Research Center.
By targeting the lipid envelope rather than the viral protein inside, tetherin can antagonize a relatively wide range of viruses, such as HIV, Ebola and others with similar envelopes. Evolutionarily, this is a clever antiviral strategy, because the viral lipid envelopes are relatively invariant while virus proteins can mutate to avoid defenses more readily, Bieniasz says. “Tetherin presents a challenging obstacle: It cannot be evaded by just changing the sequence of the viral protein to avoid an interaction,” he says. “It is a more difficult genetic step for the virus to invent an entirely new gene product to interfere with this cellular function.”
But many viruses have accomplished just such an evolutionary feat. HIV-1, for example, produces a protein called Vpu, which appears to block tetherin from taking hold in the lipid envelopes of budding viral particles. Bieniasz says a better understanding of how tetherin and Vpu interact could provide clues for a pharmacological agent that interferes with Vpu’s activity, enabling tetherin to curb the spread of HIV. Another possibility: artificial tetherin. Vpu failed to inhibit the protein researchers engineered to mimic this immune system leash.
 (opens in new window) (opens in new window) |
Cell 139(3): 499–511 (October 30, 2009) Tetherin inhibits HIV-1 release by directly tethering virions to cells(opens in new window) David Perez-Caballero, Trinity Zang, Alaleh Ebrahimi, Matthew W. McNatt, Devon A. Gregory, Marc C. Johnson and Paul D. Bieniasz |