Aaron M. Johnson
B.A., Gustavus Adolphus College
Studies of ATP Site Function in Clamp Loader Complexes
presented by Sidney Strickland (on behalf of Michael O’Donnell)
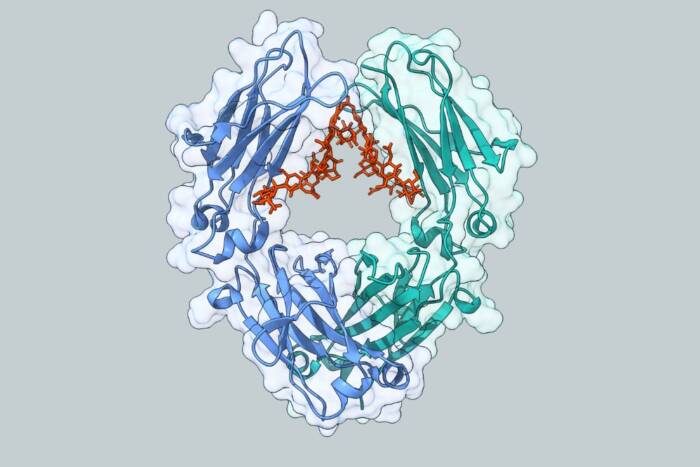
All laboratories should be so lucky as to have a student like Aaron Johnson. Not only did Aaron develop into a brilliant independent scientist, but he is a very gentle and warm spirit that added to the well-being of the laboratory every day. I am sure everybody in the lab would agree. Aaron reached another milestone in his life last July when he got married to his soul mate, Valerie Epstein. Aaron made many important scientific contributions during his graduate studies. They all centered around the mechanism by which multiprotein clamp loaders harness the energy of ATP binding and hydrolysis to open their corresponding circular protein clamp and assemble it around DNA. The circular clamp then slides on DNA and tethers the replicase to DNA for highly processive DNA synthesis. Aaron developed his interest in DNA replication mechanisms through an undergraduate project in John Kuriyan’s lab when John was here at Rockefeller University. Aaron started out in my lab as a graduate student studying the clamp loader of Escherichia coli, and the project nicely complemented the crystal structure analysis that was going on simultaneously in our collaboration with John Kuriyan’s laboratory. Aaron made specific amino acid replacements at key positions in the nucleotide binding sites and reconstituted the multisubunit clamp loader apparatus using various mutant subunits. His studies showed that the different ATP sites play distinct roles in the clamp loader mechanism.
The E. coli clamp loader contains three subunits that are identical, and this precluded developing mutants at every single specific ATP site. So Aaron took two different approaches to get around this problem. In one approach, he used expression vectors with genes that were fused together to express a large fusion protein that assembled with other subunits and placed each subunit in defined positions. This approach allowed him to place mutations in individual ATP sites of the identical subunits. The other approach he took was to study the eukaryotic clamp loader, called RFC, which has the same architecture as the bacterial clamp loader except that each subunit is encoded by a separate gene and Aaron made individual ATP site mutations corresponding to each unique site. The project led to a highly detailed understanding of the different functions of the multiple ATP sites within both the bacterial and eukaryotic clamp loaders. Of course as with any good project, the study has opened up many new interesting questions that we continue to pursue in the laboratory today.
Aaron is now performing his postdoctoral studies in the department of cell biology at Harvard University, working in the laboratory of Danesh Moazed. I’d like to mention that at the time Aaron was in my lab, there was also a postdoc named Erin — it has been great having two “Aaron Spellings” in the lab. I know I speak for the whole lab when I say that we miss Aaron very much, and we all wish him the very best in his new studies.