Rendering of ion channel suggests how neurons fire
Four years ago, Roderick MacKinnon, head of the Laboratory of Molecular Neurobiology and Biophysics at Rockefeller University, together with several members of his lab, published the first ever structure of a voltage-dependent potassium ion channel — a protein that controls the flow of potassium ions across nerve cell membranes and opens and closes in response to changes in cell membrane voltage. At that time, MacKinnon and his colleagues proposed that a structure called the paddle senses the membrane voltage, enabling the channels to open and close.
 (opens in new window)
(opens in new window)
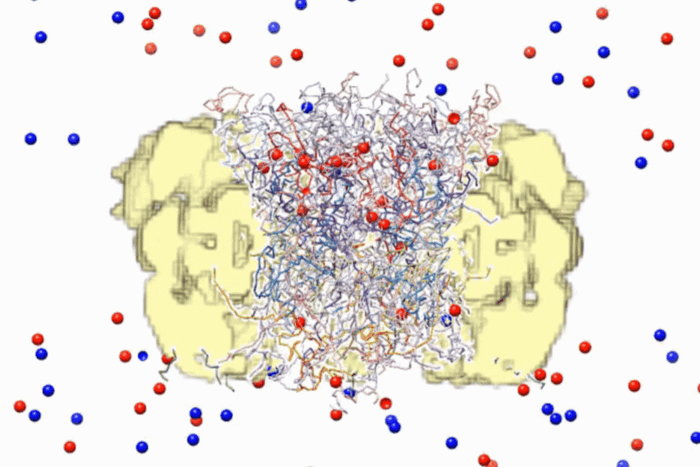
Changed channel. Lipid molecules (yellow and red) are an integral part of the voltage-dependent ion channel and confer stability to it. MacKinnon's structural renderings show that lipid molecules are most dense in the concave semicircles between the voltage sensors.
In a November issue of Nature he and lab members Steve Long, Xiao Tao and Ernest Campbell released new renderings of these channels that describe a possible mechanism for how the paddle carries its charged cargo through the channel as well as how the charged paddles are stabilized within the cell’s membrane.
Along with his colleagues, MacKinnon, a Nobel laureate and an investigator at the Howard Hughes Medical Institute as well as the university’s John D. Rockefeller Jr. Professor, took advantage of the paddle’s unique transplantability to create a hybrid ion channel — one of only a handful of eukaryotic membrane proteins whose structures have been determined through recombinant expression.
In the presence of lipid molecules, the hybrid ion channel formed a crystalline lattice that enabled MacKinnon and his team to describe the structure with unprecedented atomic detail. Within the crystals, the lipid molecules organized themselves around the several ion channel proteins, forming a lipid bilayer that mimicked the channel’s natural environment. Knowing how the lipids organized themselves around the ion channel, the researchers were then able to demonstrate how positively charged amino acids within the voltage sensor’s paddle remained stable despite the potentially destabilizing forces surrounding them. The structure also shows how movement of the gating charge across the membrane directly influences the opening and closing of the pore’s gate.
Voltage-dependent ion channels are important therapeutic targets for future treatment of pathological conditions such as epilepsy and cardiac arrhythmia. The new methods being developed for eukaryotic membrane protein structure determination, particularly in a native membrane environment, promise to advance our understanding of these therapeutic targets.
Nature 450(7168): 376–382 (November 15, 2007)(opens in new window)