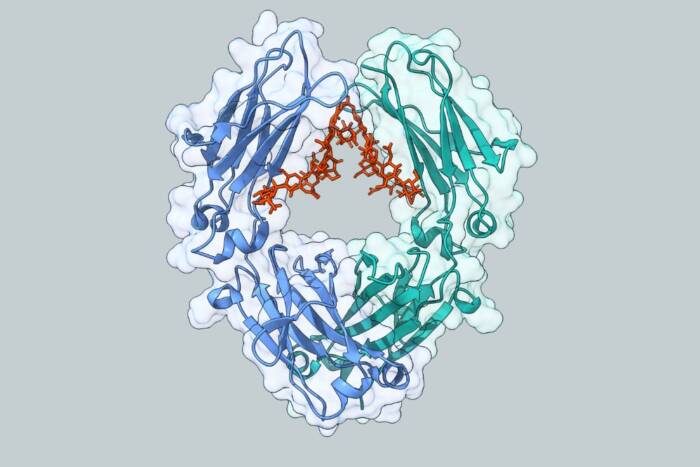
First-ever images of a living immune structure shows B cells in action
When an infection strikes, B cells act as the immune system’s tag-and-release team, hunting down the invading pathogen with incredible accuracy and labeling it with antibodies that inform other immune cells to destroy it. B cells are taught to recognize their prey inside tiny structures called germinal centers, which form within a mammal’s lymph nodes and spleen shortly after the infection sets in. Now, Rockefeller University researchers have found a way to peer inside the germinal centers of living animals for the first time, and have discovered that these training centers operate not as closed factories, as most scientists believed, but as open, dynamic systems through which B cells continually pass.
Our bodies contain a large repertoire of B cells, each one with a receptor that’s specific to a particular pathogen — so when a particular invader enters the body, at least some B cells are already equipped to recognize it. The problem is that their receptors typically have only a mild, or at best moderate, affinity for the pathogen. That’s where germinal centers come in. The structures develop when a follicle inside the lymph node is seeded by just a small number of not-yet-specialized, naïve B cells that have at least some ability to recognize the pathogen. Inside the follicle the B cells encounter antigen from the invader, and by rapidly multiplying and randomly mutating, they grow increasingly specialized to fight it. Only those with the highest affinity for the antigen survive, proliferate, and then enter the blood stream to hunt down the pathogen.
Prior studies on the germinal center seemed to indicate that once it’s seeded with the original population of B cells, the structure closes itself off and doesn’t allow any more B cells to enter. But new research by Michel Nussenzweig, Rockefeller’s Sherman Fairchild Professor, a Howard Hughes Medical Institute investigator, and head of the Laboratory of Molecular Immunology, along with Tanja Schwickert, a graduate student in the Nussenzweig lab, and collaborators at New York University School of Medicine, shows that germinal centers are not closed at all but are, in fact, open, dynamic systems.
Using two-photon microscopy, an imaging technique that can see inside living tissue, the scientists gathered the first-ever images of thriving germinal centers inside live mice. When they looked at their data, published today in an advance online publication in Nature, they were surprised to find that even as the structures cultivate a growing population of the specialized memory B cells, they also allow naïve B cells to flow through and test their antigen affinity. “Naïve B cells can go through, test their receptors, and if they have a high-affinity receptor for the antigen they can join the reaction,” Schwickert says. “This increases the competition, and the outcome is B cells that bear a very high-affinity receptor.”
As a result, Nussenzweig says, “the germinal center is not only serving as a place where you see clonal expansion, but it’s also serving as a place to hold onto the antigen and allow cells in the immune system that are transiting — and they’re transiting all the time — to see it.” So if a B cell with high affinity for a pathogen doesn’t get selected for rapid division its first time through, he notes, the germinal center’s open structure is a safety net, “a way of selecting for high-affinity cells that didn’t make it on the first go round.” By showing how B cells are given a second chance, the research explains the system’s efficiency and may offer hints to how autoimmune diseases progress.
Nature online: January 31, 2007(opens in new window)