How toothless mock viruses could advance research on COVID-19
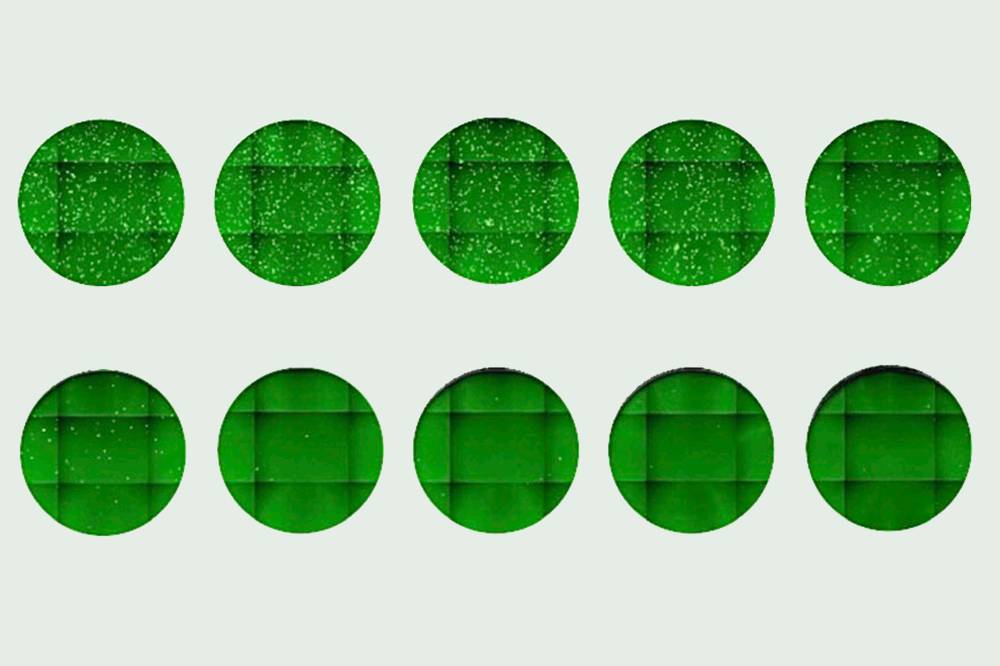
A newly discovered antibody was shown to block a pseudo-coronavirus from entering cells. Increasing its concentration (left to right, top to bottom) yielded fewer infected cells, seen as dots.
In more ways than one, antibodies could be the key to alleviating the COVID-19 crisis. Hundreds of labs around the world are studying the antibodies people produce in response to SARS-CoV-2 infection in hopes of developing treatments, predicting the efficacy of vaccines, and understanding whether those who recover from the disease are protected from a second infection.
There is a major hurdle, however: Testing the potency of these antibodies is a labor-intensive procedure that must be done under very strict safety protocols since it involves growing the highly-infectious virus in the lab. Without ways to assess antibodies more quickly and safely, progress will be slow.
Recently, scientists at Rockefeller developed a handy workaround—a set of methods to conduct SARS-CoV-2 antibody testing without using the real virus. In the Journal of Experimental Medicine, the team describes four ways to create a harmless “pseudo-coronavirus” capable of serving as a surrogate for the real thing. Taken together, these methods can facilitate the work of researchers with a range of expertise and resources.
To create the surrogate viruses, the team used either a form of HIV or vesicular stomatitis virus. They rendered these viruses unable to replicate, and further tweaked them so that they display the SARS-CoV-2 spike protein on their surface instead of their own surface proteins. “We basically decorate another virus with the spike protein, which is what the coronavirus uses to gain entry to the cells,” says Theodora Hatziioannou, a research associate professor at Rockefeller who co-directed the study with Paul Bieniasz, head of the Laboratory of Retrovirology.
The resulting virus mimics the real thing but is much safer to work with. The researchers can mix it with antibodies or plasma samples from recovered patients, and measure how well this mixture infects human cells in a dish. The better the antibodies or a plasma sample is at inhibiting virus entry, the lower the infection rate will be. “Our measurements from each of the surrogate virus-based assays correlated well with what we measured using the authentic SARS-CoV-2,” Hatziioannou says.
In just a few weeks, the team has used these assays to determine the neutralizing potencies of hundreds of plasma samples and purified antibodies in a standard biosafety level 2 laboratory (research with real SARS-CoV-2 must be done in a biosafetey level 3 lab). The assays, the researchers add, can also be used to assess vaccine-induced antibodies, providing a fast and streamlined method to gauge the efficacy of the numerous vaccine candidates currently in the development pipeline.




