Leprosy bacteria lead to new understanding of nerve damage and cell proliferation
For nervous system cells, specialization is a one-way street. But as is often the case in biology, the rules have exceptions. Glial cells — nervous system cells that form a highly specialized insulating sheath called myelin that surrounds nerve fibers — under certain conditions can “de-differentiate,” re-enter the cell cycle and revert to an unspecialized state, from which they can repair damaged tissues. Scientists are eager to learn how glial cells accomplish this trick, as it could teach them not only about neurodegenerative diseases, but also have implications for regenerative medicine.
 (opens in new window)
(opens in new window)
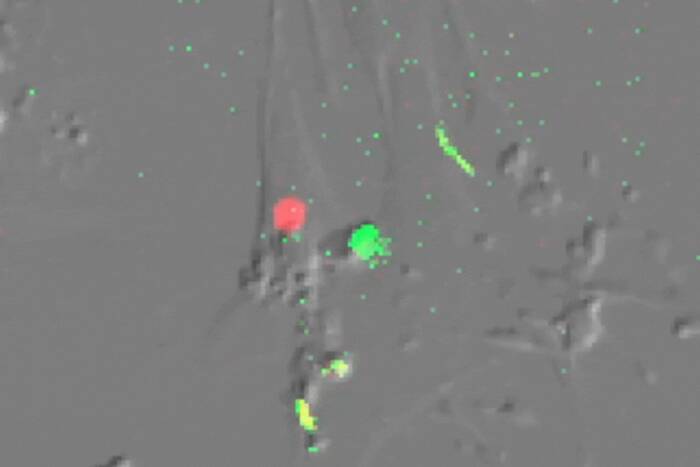
Shedding their sheath. Cells infected with the leprosy bacterium offer new clues to early demyelination in neurodegenerative diseases and cell proliferation, processes that are triggered when the bacteria activate a receptor called ErbB2 (red) on the surface of Schwann cells (green fibers and blue nuclei).
Now, using leprosy bacteria, laboratory primary cultures of human and rat glial cells and genetically engineered mice, a team of Rockefeller University scientists has uncovered new molecular pathways that lead to demyelination — the breakdown of the myelin sheath — as well as de-differentiation and cell proliferation of mature Schwann cells, the glial cells of the peripheral nervous system. Reporting in the journal Nature Medicine, the Rockefeller scientists show howM. leprae, the bacterium that causes leprosy, transmits signals from the exterior of Schwann cells, inducing demyelination and cell proliferation.
“Identification of a signaling mechanism for early demyelination has important implications not only for leprosy nerve damage but also for demyelinating neurodegenerative diseases such as multiple sclerosis, that result from a loss of a glial cell’s myelin sheath,” says principal investigator Anura Rambukkana, a research assistant professor at Rockefeller.
In the Nature Medicine paper, Rambukkana and his colleagues Nikos Tapinos and Makoto Ohnishi show how leprosy bacteria activate the classical Erk1/2 signaling pathway, which usually regulates a wide variety of cellular functions, ranging from cell proliferation and embryonic development to tumor formation and memory functions. The leprosy bacterium M. leprae activates this important pathway by adhering to the outside of Schwann cells and binding directly to a membrane receptor called ErbB2, which has been shown to be crucial for breast and ovarian cancers. Herceptin, an antibody that blocks ErbB2 and inhibits the uncontrolled cell proliferation that defines cancer, is currently being used as an effective therapy for patients with breast cancer.
Normally, in glial cells, such as Schwann cells, the ErbB2 receptor is activated by pairing with other members of the ErbB receptor family. In Schwann cells, that partner receptor is ErbB3. When neurons interact with Schwann cells, a class of neuron derived growth factors called neuregulins bind to ErbB3, which pairs with and activates ErbB2 and subsequently generates signaling cascades, one of which is the Erk1/2 pathway. To determine if M. leprae uses ErbB3 or an alternative pathway to activate ErbB2, first author Tapinos, a research associate in Rambukkana’s group, knocked down ErbB3 in primary Schwann cells. To the scientists’ surprise, M. leprae activates ErbB2 in an atypical manner that does not involve pairing with ErbB3, yet induces excessive Erk1/2 signaling. This M. leprae-induced Erk1/2 signaling — through direct activation of the ErbB2 receptor — is sufficient to cause the breakdown of the myelin sheath, which is responsible for rapid nerve conduction.
“Our studies suggest that ErbB2 or similar receptor tyrosine kinases at the glial cell membrane may serve as key sites for initiating signaling for early demyelination,” says Rambukkana.
It is now known that demyelination is also linked to the loss of all or part of neuronal functions because of the intimate connection between myelinated cells and axons. Therefore, the findings described in the Nature Medicinepaper may have implications for the development of therapies that block ErbB2 activation or diagnostic tests for detecting early demyelination before further neurodegeneration progresses.
For example, Herceptin prevents leprosy bacteria-induced ErbB2 activation and subsequent activation of demyelinating Erk1/2 signaling in human primary Schwann cells. Also, the researchers showed that a small molecule inhibitor of ErbB2, PKI-166, which is currently being used in cancer trials, significantly reduced bacteria-induced early demyelination in mice with compromised immune systems, indicating that this pathway is operating not only in tissue cultures, but in the living mouse.
In an accompanying News and Views article in the same issue of Nature Medicine, Robert Franklin and Chao Zhao, experts in glial cells of the brain at the Center for Brain Repair of the University of Cambridge, UK, discuss the potential of this finding to gain more general insights into the biology of other peripheral nerve diseases and demyelinating central nervous system diseases. The Rockefeller team’s research, the authors write, ”reveals several points at which the process can be blocked and demyelination reduced.”
The Nature Medicine study complements research that the same team reported a year ago in Proceedings of the National Academy of Sciences, where the Rockefeller researchers showed that leprosy bacteria that had entered Schwann cells activated Erk1/2 by a novel, non-classical pathway called PKC/LCK, leading to continuous cell proliferation without transformation into cancer cells. Identification of new pathways that activate Erk1/2 now allows scientists to discover new roles for Erk1/2 signaling in many vital cellular functions. Rambukkana’s team is focusing on the role of these newly identified Erk1/2 pathways in mammalian glial cell and stem cell biology.
“The ability to trigger cell survival and proliferation signals is a unique feature of the leprosy bacterium, which is non-toxic, because most other bacteria cause cell cycle arrest, apoptosis or kill the cells within a short period or at some point during infection,” says Rambukkana.
When leprosy bacteria are inside adult Schwann cells, the bugs eventually reset the cells to a highly immature or stem cell-like stage as a result of continuous proliferation and de-differentiation. This persists despite the fact that in time the cells grow much faster than the bugs — the estimated dividing time for M. leprae in vivo is once every 14 days — and eventually the cells no longer contain the bacteria. The findings from the two papers show that both of these new pathways of Erk1/2 activation lead to Schwann cell propagation and de-differentiation. From the bacterial point of view, this is a critical requirement for initial colonization of strictly intracellular leprosy bacteria within the peripheral nervous system.
“The finding that leprosy bugs have the capacity to induce cell proliferation indicates that these bugs can rejuvenate the cells they infect,” says Rambukkana. “We hope to use these very unique bacterial properties to dissect the basic biology of mammalian glial cells and to understand how adult Schwann cells are de-differentiated and reprogrammed. That knowledge can then be used in efforts to further regenerative medicine.
Nature Medicine 12: 961-966 (August 1, 2006)(opens in new window)