By targeting dendritic cells, HIV and malarial vaccines outperform competitors
Although DNA-based vaccines are often in the limelight, scientists at Rockefeller University are developing a completely different approach to inducing immunity, one that directs a vaccine straight to the immune cells of living animals and, eventually, humans.
 (opens in new window)
(opens in new window)
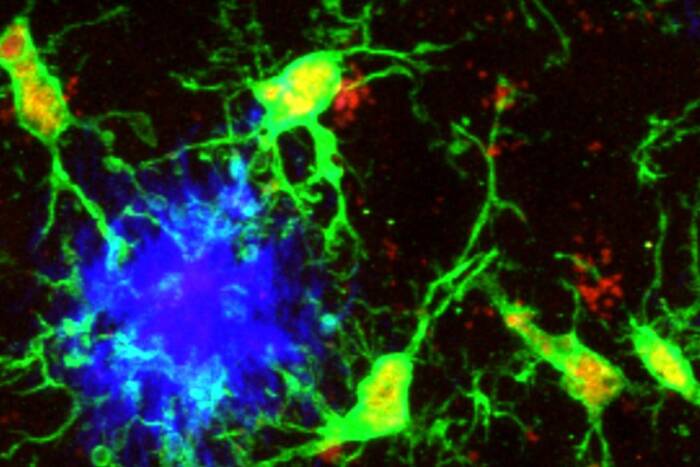
Harnessing dendritic cells. Dendritic cells direct the body's immune response, making them an ideal vaccine target. Using a known receptor on their surface (the DEC-205 receptor, shown here in red in the dendritic cells of a human lymph node), Rockefeller researchers have begun, in mice, to develop a new approach to vaccines against malaria and HIV.
In two papers published this month in the Journal of Experimental Medicine, Ralph Steinman, Henry G. Kunkel Professor and head of the Laboratory of Cellular Physiology and Immunology, Michel Nussenzweig, Sherman Fairchild Professor and head of the Laboratory of Molecular Immunology, and their colleagues show that they can target dendritic cells of living animals and induce specific defenses against malaria and HIV that appear to outperform current methods.
These new findings build on research published a few years ago by the two collaborators, in which they showed that it was possible to deliver an immunity-stimulating antigen directly to dendritic cells in living mice. These dendritic cells, which Steinman co-discovered in the 1970s, are responsible for directing immune response, and have the potential to greatly increase the efficiency with which the immune system is engaged. To determine whether antigen delivery translates into improved immunity, the researchers tested their method on two dramatically different kinds of invaders: Nussenzweig, an HHMI investigator, worked on malaria and Steinman concentrated on HIV. “We were interested in two things: Could we extend this discovery to some clinically interesting antigens and, if so, were there hints that it could be better for vaccine design?” says Steinman.
Now, the scientists have their answer. By targeting a known antigen receptor on the dendritic cells, called DEC-205, they were able to direct both malarial and HIV proteins straight to them. This, in turn, produced a strong response by CD4+ helper T cells, immune cells that assist in the creation of antibodies that defend against specific foreign invaders. “We knew dendritic cells could induce helper cells, but we had no idea how strong the helper response would be, and how broad it would be in terms of the number of microbial peptides that were recognized,” Steinman says.
The malaria paper, by first author Silvia Boscardin, a postdoctoral associate in Nussenzweig’s lab, examined how effectively the malarial protein, known as circumsporozoite protein or CSP, induced T-cell function. When they injected the CSP vaccine into three separate strains of mice, they found that a single injection was enough to elicit robust antibody and cell-mediated immune responses in all three mouse strains.
In the HIV paper, Christine Trumpfheller, a research associate in Steinman’s lab, delivered an HIV protein called gag to the dendritic cells. This, too, induced a broad-spectrum immune response in the three different mouse strains. And when the researchers compared their method to other existing experimental HIV vaccines, they found it had a considerable advantage: Both a DNA-based vaccine, which is already in human trials, and an adenovirus vaccine, which uses a deactivated cold virus to infect cells with antigen, fell far short of the CD4+ helper response produced by the gag vaccine. But, the adenovirus solution did stimulate the production of a large number of killer T cells (which, as their name suggests, attack and kill invading cells), something the DEC vaccine did not. “This is all in its very early stages, but maybe we should be combining the adenovirus and the DEC approaches,” says Steinman, who’s also a senior physician at the Rockefeller University Hospital.
Current vaccine methods don’t directly target dendritic cells. But the DEC vaccine goes straight to them, right to the source of immune system activation. “This is a totally new and very logical approach for vaccine design,” Steinman says. And it provides a much less complicated way to study what dendritic cells are doing in a living animal, because it can measure the effects of a vaccine targeted to immune organs, rather than to cells in test tubes or tissue culture. The collaborators are now developing assays to determine how long the immunity generated by their DEC-205 vaccines lasts, and they’re seeking other dendritic-cell targets, too. “There’s still tons to do,” Steinman says. “We think many areas of immunology have to be looked at again from this perspective.”
Journal of Experimental Medicine: February 2006(opens in new window)
Journal of Experimental Medicine: February 2006(opens in new window)