How one of nature's most fundamental molecules forms
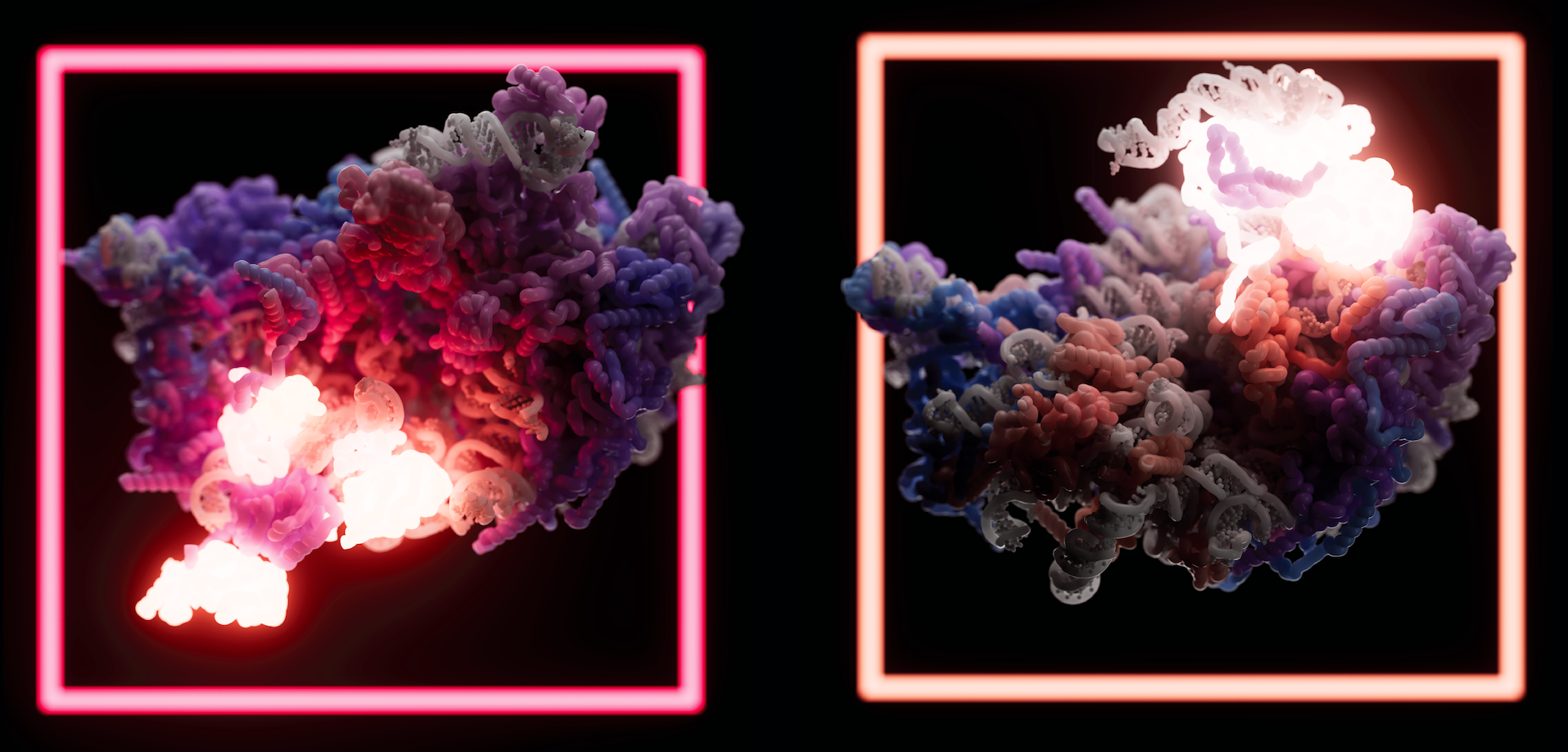
Two key stages in the formation of the human large ribosomal subunit (60S). Credit: Jeroen Claus/Phospho
Life runs on ribosomes. Every cell on earth needs ribosomes to translate genetic information into all the proteins needed for the organism to function—and to in turn make more ribosomes. But scientists still lack a clear understanding of how these essential nanomachines are assembled.
Now, new high-resolution images of the large ribosomal subunit are shedding light on how arguably nature’s most fundamental molecule coalesces in human cells. The findings, published in Science, bring us one step closer to a complete picture of ribosome assembly.
“We now have a pretty good idea of how the large ribosomal subunit is assembled in humans,” says Rockefeller’s Sebastian Klinge. “We still have quite a few gaps in our understanding, but we certainly now have a much better idea than we had before.”
Solving the large subunit
Ribosomes were first discovered at Rockefeller almost 70 years ago. Scientists have since determined that they are composed of two subunits: a small subunit, known as 40S, which is responsible for decoding messenger RNA, and a large subunit (60S), which attaches protein pieces together. But those were the very broadest strokes. The precise steps by which these complex molecules are assembled into their mature form has long remained a mystery.
Klinge’s approach to this larger problem has long focused on figuring out how ribosomes form in the first place. To that end, Klinge’s lab was among the first to use cryo-electron microscopy to capture footage of a nonbacterial ribosome assembling towards its final shape, and the lab has since taken an even more granular approach—painstakingly stringing snapshots of maturing ribosomes together, to understand how these molecules get from one point in their assembly to the next.
In recent years, Klinge and other scientists around the world have identified and characterized more than 200 ribosome assembly factors that influence the modification, processing, and folding of ribosomes.
For the current study, Klinge and colleagues focused on the human large ribosomal subunit (60S). The team already knew, from studies in yeast, that the large subunit’s formation involves two precursors (a 5S rRNA and 32S pre-rRNA) snapping together, but “we wanted to know all of the events that need to happen for this to occur,” says Arnaud Vanden Broeck, a postdoctoral researcher in Klinge’s lab. “We wanted to explain how the large subunit is assembled and processed in human cells.”
Vanden Broeck and Klinge combined new techniques involving a mashup of genome editing and biochemistry, to capture high-resolution cryo-EM structures of 24 human large ribosomal subunit assembly intermediates as they were maturing. The resulting images show how assembly factors, various proteins and enzymes, interact with RNA elements to drive the formation and maturation of the 60S. Together, the findings represent a near-complete picture of how the human large subunit assembles.
“For sixty years we had almost nothing on the intermediates that form the human 60S—it was all but invisible to us—and now we’ve jumped from nothing to pretty good coverage,” Vanden Broeck says, while admitting that some of the rarest and most transient steps on the road to the mature 60S may have evaded the team, and fallen through the cracks. “We still have a lot of work to do.”
Nonetheless, key findings from the study could already begin informing related fields of inquiry. Among the intermediary steps discovered, for instance, are signaling pathways that suggest a link between ribosome assembly and cellular metabolism—suggesting that a complete understanding of ribosomes may well require close collaboration with experts in cell metabolism. And the granular look at the steps of ribosome formation provided by the study may provide important context for scientists studying diseases linked to ribosome mutations.
For now, however, Klinge and Vanden Broeck are content to marvel at the substantial leap forward. “It’s not guesswork anymore,” Klinge says. “We can now see, in detail, what’s going on when the large subunit assembles. It’s humbling to realize we’re finally able to see what makes ribosomes and drives protein formation in all of our own cells.”



