Intriguing science discoveries of 2023
 This year, Rockefeller scientists plumbed the depths of wound repair and tackled how songbirds solve problems; they used microchips to grow mini-lungs and proposed an environmental trigger for multiple sclerosis. Efforts to combat COVID, Hepatitis B, and other infections bore fruit, and countless papers shed light on basic research, answering questions that have long baffled biologists. Here are some of the intriguing discoveries that came out of Rockefeller in 2023.
This year, Rockefeller scientists plumbed the depths of wound repair and tackled how songbirds solve problems; they used microchips to grow mini-lungs and proposed an environmental trigger for multiple sclerosis. Efforts to combat COVID, Hepatitis B, and other infections bore fruit, and countless papers shed light on basic research, answering questions that have long baffled biologists. Here are some of the intriguing discoveries that came out of Rockefeller in 2023.
As the male reproductive system ages, it becomes more and more susceptible to mutations. New research from the laboratory of Li Zhao explored this phenomenon in fruit flies, by focusing on how mutations arise during the formation of sperm. The team found that, while mutations are common in the testes of both young and old flies, the repair mechanisms that remove those mutations and maintain genomic integrity during spermatogenesis become less efficient in older individuals, leading to the accumulation and persistence of more mutations in older flies.
The findings could ultimately help researchers understand how older human dads impact the genetic health of their offspring. But the team intends to first better characterize their fly-related findings. “What genes are really driving the difference between old and young flies in terms of mutation repair?” wonders Evan Witt, a former graduate student in the lab and now a biologist at Biomarin Pharmaceuticals.
Scientists colloquially call the body’s first encounter with a virus an “original antigenic sin” (OAS) that forever biases the immune response against newer strains. No matter how many flu vaccines or COVID boosters we receive, the theory of original antigenic sin dictates that our bodies will stubbornly churn out antibodies tailored to the first strain we encountered.
A new study from Gabriel D. Victora’s lab may have discovered a way around OAS, with findings suggesting that a booster shot containing an antigen sufficiently different from that of the original infection can reset the body’s immune response to its factory settings.
Although the work was conducted in mice, the team suspects that the same rules may apply to human booster shots—and that boosting humans against a new strain may be most effective when the new strain is sufficiently different from the one covered by the previous vaccine. “It may well be a matter of waiting to update vaccines until the virus is sufficiently divergent,” Victora says. “That would be just the right time to develop a booster.”

There are queen ants, and then there are “workerless social parasites”—ants living as queens, mooching off the colony while in disguise. These parasites were once thought to have evolved the characteristic wings, large eyes, and ovaries that make up their queen costume through gradual mutations, but recent work from the laboratory of Daniel Kronauer suggests that queen-like mutant ants appear in colonies spontaneously. “This mutant is like the precursor to other parasitic species,” says Waring Trible of Harvard University, who conducted the research with Kronauer. “It’s a new way of understanding how ants evolve to become socially parasitic.”
The discovery sheds light on the molecular mechanisms behind ant caste development, and some of the specific findings may inform future study of the biological processes that ensure the tissues of every animal remain proportionate to its body size as it grows.
Short-term memories form in the hippocampus and, if the situation calls for it, stabilize into long-term memories in the cortex. But what happens along the winding path between short-term to long-term memory? Research from Priya Rajasethupathy’s lab now points to the anterior thalamus as the all-important brain region that determines whether a memory makes it into the archives.
“The thalamus is in continuous dialogue with the cortex over weeks, saying ‘stabilize this’,” Rajasethupathy proposes. “Less salient memories drop off because the cortex isn’t getting a constant signal from the thalamus to keep this memory.” The team suspects that this new understanding still only tells part of the story. Rajasethupathy hopes that her findings will be blended with other models, as neuroscientists continue to solve the mystery of memory.
Although antivirals can now cure 95 percent of hepatitis C infections, its cousin hepatitis B still claims a million lives each year. A team led by Nobel laureate Charles M. Rice has now developed a platform for studying hepatitis B virus in the lab. This method sidesteps the virus’s typical replication process, providing a sharper view of its behaviors during a crucial part of its life cycle. The researchers hope that this novel approach will expose heretofore unknown weaknesses in the virus, leading to the development of new therapies, or a cure, for hepatitis B.
“Anywhere you can impinge on that life cycle and prevent this virus from replicating and spreading to new cells could be a potential target for new drugs,” says Bill Schneider, a research associate in the Rice lab.
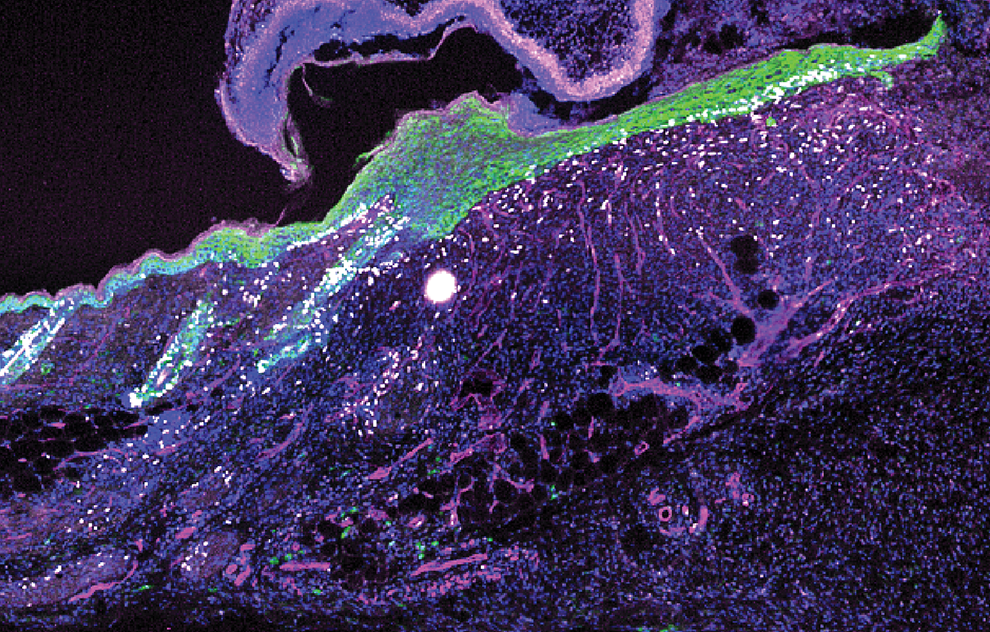
Our skin takes a beating, continually responding to threats of injury and infection. Usually it activates the immune system upon sensing pathogens, but research from the laboratory of Elaine Fuchs has identified an alternative method by which the skin protects itself: by sensing broken blood vessels, the formation of scabs, and other signs of injury.
These telltale signs of trauma kick off an ancient wound repair response, mediated by the gene interleukin-24. “IL24 becomes an orchestrator that coordinates tissue repair,” Fuchs says. The findings also highlight an evolutionary link between IL24 and immune system proteins, suggesting a common ancestral pathway that diverged to address tissue injury.
If we knew how metabolites entered cells, we could begin developing drugs to treat conditions linked to metabolite transport, from rheumatoid arthritis to neurological disease. But matching proteins with the nutrients that they shuttle into the cell has proven difficult—30 percent of carrier proteins and nutrients have yet to be matched up.
Kivanc Birsoy’s lab recently introduced a method for systematically identifying “orphan” transport proteins, allowing scientists to finally make those matches. As a proof of concept, the team used this method to discover the protein responsible for transporting choline into the cell. This finding may have immediate implications for people living with posterior column ataxia with retinitis pigmentosa (PCARP), a disease caused by a transporter mutation that affects vision and the nervous system. “Our findings could be easily translated into the clinic,” says Timothy Kenny, a postdoc in the Birsoy lab.
From microchips to “micro lungs”
The laboratories of Ali Brivanlou and Charles M. Rice have developed a platform that uses microchips to grow lung buds from human embryonic stem cells. These stripped down “micro lungs” have all the complexity of lung tissue without any of the molecular frills that make individual lungs different from one another, allowing scientists to drill down into how diseases impact lung tissue, free from distractions. These identical micro-lungs can also be cultured by the thousands, allowing for an unprecedented high-throughput analysis of lung tissue infection.
The researchers hope to use the micro lungs to investigate the mechanisms of COVID, influenza, RSV, pulmonary diseases, and lung cancer—and to screen new therapies to treat them. “The platform will also allow us to respond to the next pandemic with much more speed and precision,” Brivanlou says.

Only a handful of animal groups are capable of complex vocal learning, defined as learning and retaining a large number of sounds. Humans, elephants, whales, seals, bats, songbirds, parrots, and hummingbirds are among the most famous, and a recent study from Erich D. Jarvis’ lab suggests that complex vocal learning may go hand in hand with superior problem-solving skills.
“Complex vocal learners should also be better at cognitive tasks, but no one had ever demonstrated that before,” says Jean-Nicolas Audet, a research associate in the Jarvis lab.
The team spent three years catching hundreds of wild birds from 21 species in mist nets at The Rockefeller University Field Research Center, a sprawling 1,200 protected acres of land compromising many different ecosystems in New York’s Hudson Valley. They found that starlings, blue jays, and catbirds are the most advanced vocal learners and also the most adept at solving puzzles—suggesting that complex vocal learning is indeed linked to intelligence.
Scientists have long suspected that multiple sclerosis is precipitated by an environmental trigger; likely some sort of microbial infection. The question is which microbe to blame.
Now, new research from Vincent Fischetti’s laboratory suggests that a toxin produced by a C. perfringens—a common microbe found in sewage, marine sediment, soil, and the GI tracts of pets and farm animals—can trigger the inflammation characteristic of MS in mice.
Whether these findings will bear out in humans remains to be seen. “If this is the environmental trigger for MS, we can now start talking about a vaccine, monoclonal antibodies, or some other therapy,” says Rashid Rumah, a physician scientist in the Fischetti lab.


