A new way of thinking about how organ architecture develops
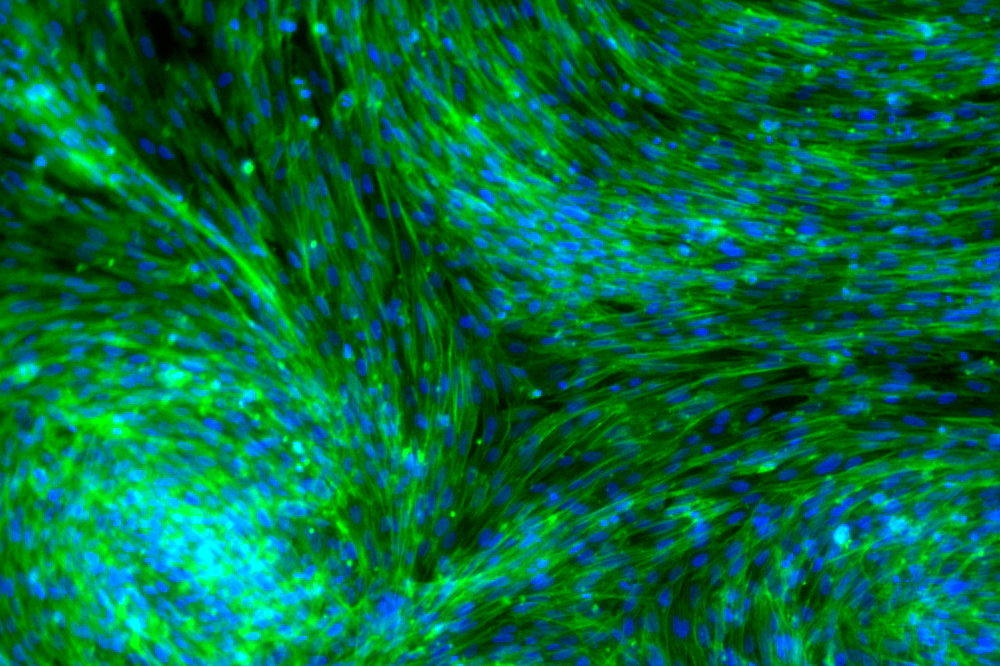
Fluorescence light micrograph of fibroblasts derived from embryonic chicken skin display collective order.
Within every developing embryo lies the mystery of self-organization: how does an organism go about shaping itself even while it’s in the process of making its parts?
By employing a holistic rather than reductionist approach to the study of tissue formation, researchers have revealed how signaling molecules influence the biophysical processes that shape the developing organ. These processes are described in a new study in (opens in new window)Science led by Alan Rodrigues and Amy Shyer, co-directors of the Laboratory of Morphogenesis at The Rockefeller University.
Underlying this work is a fundamental shift in mindset that seeks to provide a broader, sense-making context for the study of organ development, one that may even translate to more effective diagnosis and treatment of many human diseases.
“The question that drives much of our work is how thousands of cells come together to generate the ordered patterns seen in tissues,” says Rodrigues. “Our results indicate that there is an emergent order beyond molecules and individual cells that needs to be taken into account.”
A bird’s eye view of organ development
Prior work on organ development has leaned heavily on morphogens, signaling molecules that play a pivotal role in influencing cell identity and behavior. Seminal studies have demonstrated that various morphogens, when mutated, can lead to malformed organs (work that resulted in a Nobel Prize(opens in new window)).
But most work on morphogens has focused on how these signaling molecules function at the genetic or molecular level, cell by cell, under the assumption that the complex choreography of tissue formation comes down to the genes expressed in each individual cell. Rodrigues and Shyer suspected that a broader look at entire groups of cells destined to form tissue—a view from the so-called collective cell scale or supracellular level—could go a long way towards explaining what has heretofore remained inexplicable. “Morphogens are critical to development, but there are many unanswered questions about how they participate in shaping tissues, especially when collectives of thousands of cells are involved,” Shyer says. “We sought to think beyond the individual cell and look at tissue formation on a larger scale.”
To demonstrate the importance of this bird’s eye view of organ development, Rodrigues and Shyer turned to the developing chicken skin as a model. The researchers chose their system for its ideal level of complexity: skin develops as a flat sheet dotted by an array of bumps, which means it’s simple enough to work with yet complicated enough to stand-in as a model for the intricacies inherent to human organs. By investigating skin embryonic tissue beyond the scale of a cell, the team hoped to identify how new structures are created in an organ.
The structures of skin come together during a critical window at about a week into chicken embryo development, analogous to about a month into human development in utero. “It’s a unique and pivotal stage, when a human is almost indistinguishable from a cow, mouse, or chicken. This is when you get the tissue architecture that persists throughout your life, ” Rodrigues explained. “Vertebrates look remarkably similar at this stage, suggesting that deep, conserved principles are present.”
Viscosity, elasticity, and mechanical activity
After identifying key inflection points in the development of embryonic skin, the team began analyzing it at the collective cell scale. The authors focused on characterizing shifts that emerge in the material and mechanical properties of dermal cell collectives when exposed to morphogens: viscosity, elasticity, and mechanical activity.
Focusing on collectives of cells, rather than individual cells, offers a way to observe functionally meaningful characteristics that would otherwise be missed. A single cardiac cell can’t pump blood and a single neuron can’t write an opera; the heart and brain only fully function through collective action that somehow surpasses the capabilities of its constituent units. Those who study such complex systems call such phenomena emergent properties, because these abilities don’t reside within any single component, but only emerge through their dynamic interrelationships.
The team hypothesized that emergent properties of cell collectives could be reflected in physical properties such as viscosity, elasticity, and mechanical activity. “The challenge we wanted to tackle was to experimentally capture and therefore argue for the existence of these emergent properties at the collective cell level,” says Rodrigues.
Together with co-first authors Sichen Yang and Karl Palmquist, who both conducted their graduate studies at Rockefeller, the team developed techniques to measure supracellular physical properties. One assay involved atomic force microscopy, which tests the material properties of a tissue at large by prodding it with a probe and measuring its hardness. “What’s nice about the collective cell scale is that you can actually pull and push it,” Shyer says. “It’s a very tactile way of doing things.”
The team also used another assay, called spheroid fusion, where they characterized how two “spheroid” clusters of cells fused together when placed next to each other. “When two raindrops come into contact, they fuse quickly into one large droplet, indicating their fluidity. On the other hand, when two billiard balls are placed next to each other, they remain separate, indicating their solid nature,” says Shyer. When spheroids were treated with one specific morphogen, bone morphogenetic protein (BMP), the cell clusters fused together like water. However, when spheroids were treated with a different morphogen, fibroblast growth factor (FGF), they only partially merged like two clay balls, indicating increased solidity.
Then, working with Pearson Miller, an applied mathematician and fellow at the Flatiron Institute, the team began exploring how such shifts in supracellular physical properties might be responsible for making new structures. The team combined quantitative biophysical models with experimental data to provide evidence that a solid core surrounded by an active fluid margin emerges in a geometry that is mechanically unstable. This instability resolves itself and, in the process, creates a protrusion that rises out of the plane of the skin. Influenced by subcellular scale thinking, the field had assumed that these protrusions were based on individual cells migrating or local proliferation. In contrast, this study suggests that the key physical action responsible for creating new organ structures is at play at the supracellular scale.
“This is sufficient to generate the shape of the skin and the follicle,” Shyer says. “So, what we’re seeing is that morphogens do not directly orchestrate the sculpting of organs. Their influence is mediated through supracellular properties and processes, which is something we are only now beginning to appreciate.”
When there’s no smoking gun
Although these findings could only have emerged by taking properties beyond the single cell into account, Shyer notes that morphogens do play a key role at the cellular level. “Molecular changes do, of course, occur within each cell when treated with either BMP or FGF,” she says. Indeed, the team characterized molecular features within the cellular collectives and found key changes in the cytoskeleton as well as in extracellular matrix composition and arrangement. In addition, single-cell sequencing revealed that a single morphogen likely modifies the expression of dozens to hundreds of genes. At the same, from a material perspective, the contribution of these molecular changes manifested at the supracellular scale.
Shifting focus to the collective cell scale may have implications for human health. For instance, because a burgeoning tumor shares characteristics with an embryonic structure, the researchers are now using their method to explore cancerous growths. “Our hypothesis is that we won’t fully understand why a single mutated cell forms a tumor unless we investigate the tumor tissue at the supracellular level,” Rodrigues says. In pursuit of this lead, the team is currently looking at how their methods might inform the study of ovarian cancer.
Taking a supracellular approach may open up new modalities of disease diagnosis and treatment. “It could be the case that the subtle tuning of hundreds of genes coalesces into emergent material and mechanical properties that contribute to the breakdown of healthy tissues,” explains Rodrigues.
“The relatively confined number of potential supracellular properties may provide a much-needed foothold for treating the many disease areas where there is no single molecular smoking gun.”




