Rockefeller researchers identify new role for key protein that regulates separation of DNA in dividing cells

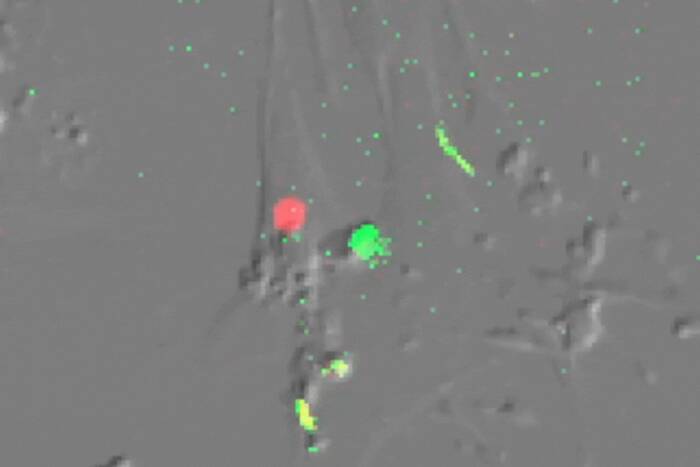
Left to right: A dividing cell with chromosomes correctly attached to microtubule spindles; incorrect attachments in a cell with BubR1 “knocked down” by RNAi; attachments “rescued” by inhibiting an enzyme called Aurora kinase. Microtubules are green, DNA is blue.
Rockefeller University scientists have revealed a new function of a key component of the mechanism that cells use to accurately separate chromosomes when they divide. Disruptions in this process can cause diseases such as cancer.
Reporting in the January issue of Nature Cell Biology, Michael A. Lampson, Ph.D., and Tarun M. Kapoor, Ph.D., show that a protein call BubR1 coordinates two pathways that the cell uses to ensure that all the chromosomes are correctly attached to structures in the dividing cell called spindle microtubules.
When cells divide the parent cell must replicate and segregate — with exquisite precision — each of its 46 chromosomes so that two “daughter” cells inherit all of its genetic information. During normal cell division, each replicated chromosome pair attaches to a bipolar structure called the mitotic spindle, made up of protein polymers known as microtubules. For each replicated pair one sister chromosome attaches to one pole of the spindle, and the other sister attaches to the opposite pole. When the cell divides, sister chromosomes split up and are pulled in opposite directions so that each daughter cell receives exactly one copy of each replicated chromosome.
When errors in this process occur, the daughter cells don’t receive all their genes, leading to developmental defects and diseases such as cancer in the organism. One such error occurs when both sister chromosomes of a replicated pair attach to the same pole of the mitotic spindle in a dividing cell. The result is that both sister chromosomes are pulled in the same direction, leading to an extra copy of a chromosome in one daughter cell and a missing chromosome in the other daughter cell.
The dividing cell uses two strategies to ensure that each daughter cell receives its full complement of genes. One such strategy involves regulating the attachments of chromosomes to each of the spindle poles, which Lampson and Kapoor showed in a 2004 paper in Nature Cell Biology is performed by an enzyme called Aurora kinase.
The other strategy is to delay the onset of a stage of the cell cycle called anaphase until all the attachments are correctly made.
“Once a cell goes into anaphase, there is no going back,” says Lampson, a postdoctoral researcher in the Laboratory of Chemistry and Cell Biology, headed by Kapoor. “It’s an irreversible step.”
A process called the mitotic checkpoint monitors the attachment process and, if it detects incorrect attachments, delays anaphase. This essentially allows the attachment machinery to correct any flawed attachments before the cell goes into anaphase. Once all the attachments are correctly made, the checkpoint shuts down and the cell cycle proceeds.
The question Lampson and Kapoor wanted to answer was: How are the two strategies coordinated?
Lampson used a technique called RNA interference to “knock down” three proteins, BubR1, CENP-E and Mad2, in laboratory cultures of human cells. BubR1 and Mad2 have been shown by other researchers to be essential for activation of the mitotic checkpoint.
Lampson found that loss of either CENP-E or Mad2 had little or no effect on the spindle attachment process. But when BubR1 was knocked down, he observed that the chromosomes were not able to position themselves because the attachments were not regulated properly.
“BubR1 is essential for both checkpoint activation and regulating spindle attachments,” says Lampson.
Lampson went on to show that in cells in which BubR1 was not functioning, disabling Aurora kinase “rescued” the cell from the BubR1 defect and allowed the chromosomes to form attachments to the spindle poles.
“The way we think about the attachment process,” Lampson says, “is that the attachments form and when they are correct, the cell locks them in that state. And when they are not correct, the cell activates machinery that corrects them.
“When we inhibit Aurora, the chromosomes are able to form attachments, but they all get locked regardless of whether they are formed correctly or not.”