New mechanism for maintaining genome stability discovered
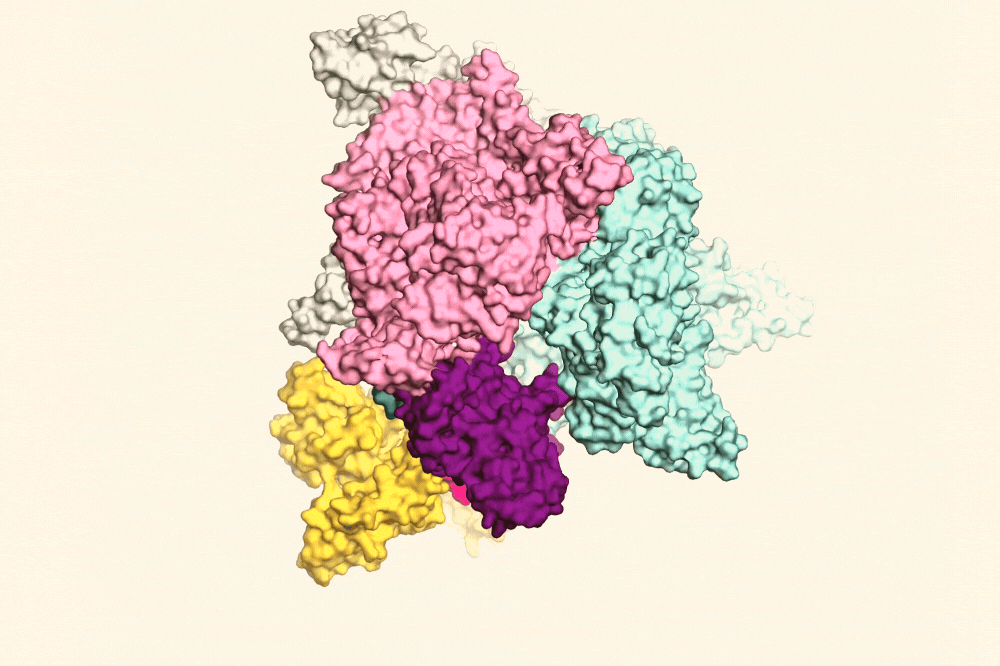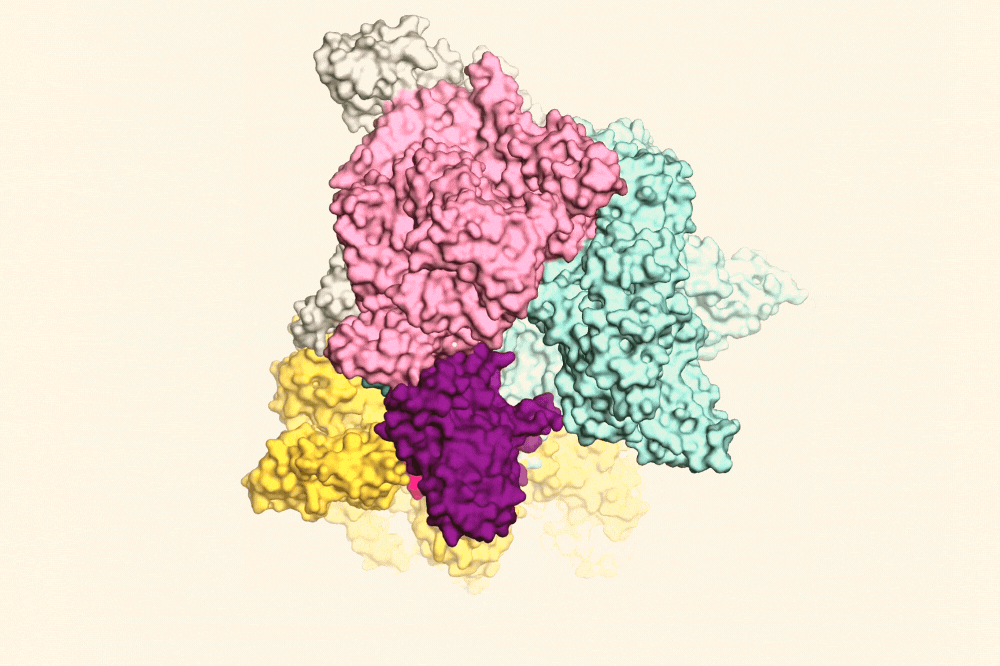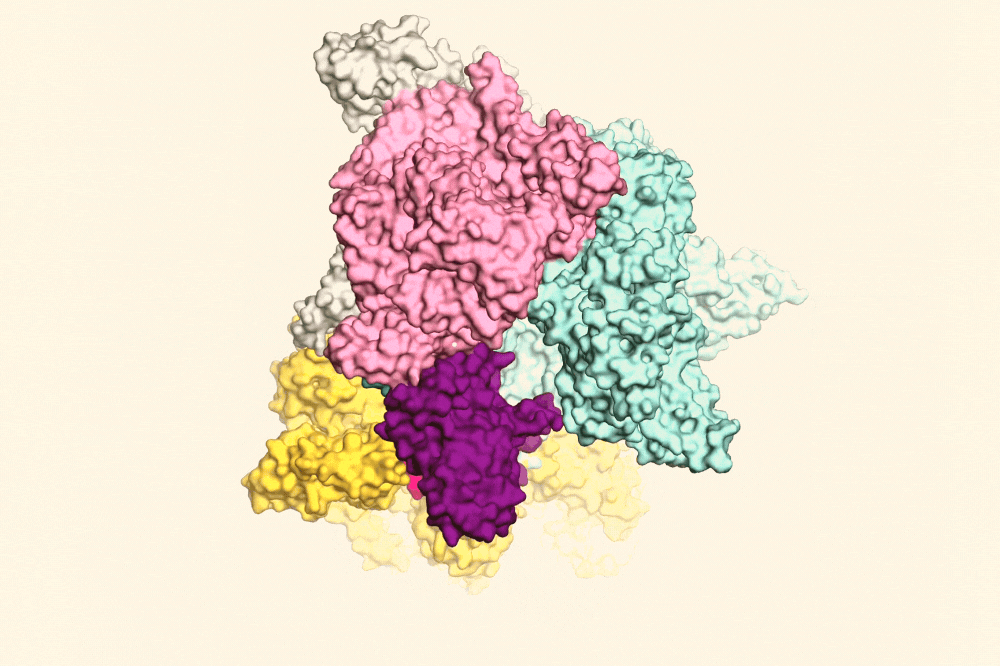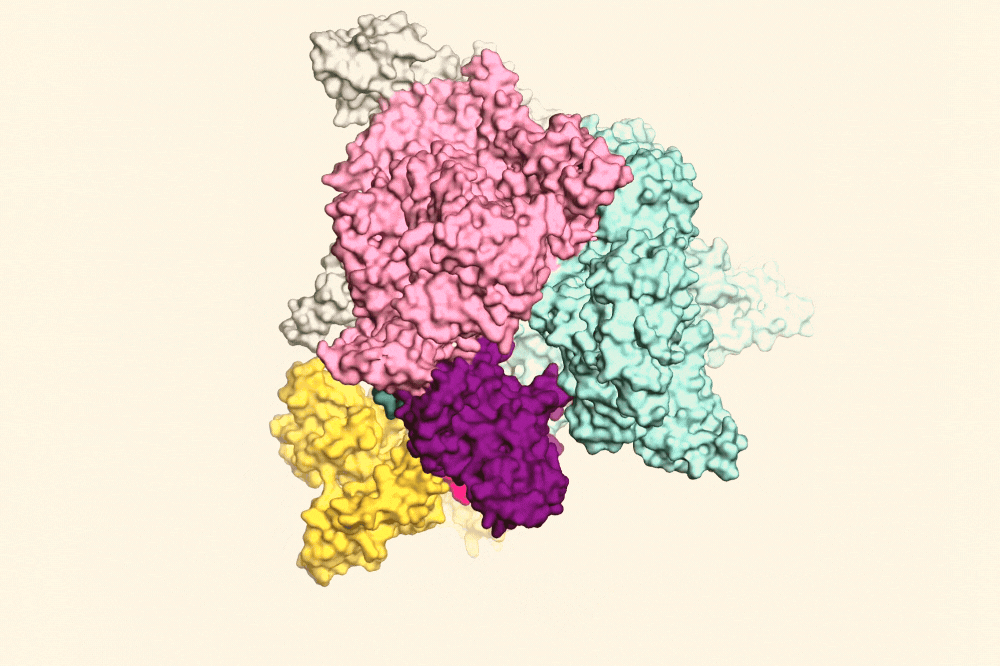
RapA (yellow) pulling open the RNAP clamp (purple) to help the RNAP fall off the DNA.
Genetically speaking, it’s a bacterium’s worst-case scenario: during transcription, newly minted RNA sticks to its DNA template, forming a 3-stranded structure known as an R-loop. While these structures have some important roles to play in a cell, R-loops in the wrong place at the wrong time can be disastrous, leading to DNA breaks, mutations, and cell death.
Now, new research in Nature Structural & Molecular Biology (opens in new window)describes how the enzyme RapA prevents R-loop formation in E. coli, with far-reaching implications for how all cells maintain genomic stability. The findings demonstrate that, under certain circumstances, the RNA polymerase (RNAP) enzyme responsible for copying DNA into RNA can generate rampant R-loops—if not for the intervention of a protein called RapA.
“R-loops are generally bad news, so cells have many redundant mechanisms to prevent them from forming,” says Seth Darst, head of the Laboratory of Molecular Biophysics. “We discovered that RapA, a protein that we’ve been curious about for years, is one of those key mechanisms.”
The jaws of life
All living things rely on RNAP to transcribe DNA into RNA. In bacteria, researchers have long known that transcription begins when RNAP clamps onto a DNA strand and initiates the process after receiving the green light from sigma proteins. But the details of how transcription ends are still fuzzy. New studies have shown, for instance, that RNAP often remains clamped to the DNA even after the newly finished RNA transcript is released—why and how was poorly understood.
Back in the 1990s, the Darst lab had discovered RapA, a ATPase that clearly interacted with RNAP—but with no obvious function. “At the time, we really couldn’t figure out what RapA was doing,” he says. But after a few decades, when a separate research group found that E. coli exposed to stressful, high-salt conditions could not grow without RapA, Darst’s interest in the mysterious protein reignited.
His team set about using cryo-EM to examine how RNAP remains clamped to DNA after transcription termination, and how RapA interacts with it. They chose negatively supercoiled DNA—an underwound and twisted form of the double helix—because it better mimics the natural state of bacterial DNA than the linear DNA often used in structural studies. “Ours is one of the first studies to use negatively supercoiled DNA in a cryo-EM experiment,” says first author Joshua Brewer, who designed the experiment. “This method helped us better visualize the topological state of the DNA, how the proteins rearrange themselves, and how they interact with the DNA.”
They were surprised to find that RNAP seldom sits idly when it remains clamped to DNA after transcription is complete. Instead, it can initiate transcription again, this time without the normal safeguard of sigma proteins. In the absence of sigma, transcription initiation forms harmful R-loops unless RapA can intervene first, prying open the RNAP clamp. “RNAP is like a big claw that closes around DNA,” Darst says. “RapA binds RNAP and pulls the clamp open so that it falls off the DNA before it can create R-loops.”
Beyond bacteria
A clearer picture of RapA’s role began to emerge. When the team subjected bacteria engineered without RapA to stressful, high-salt conditions, the microbes experienced genetic instability—evidence that RNAP is more likely to remain clamped to DNA and more likely to form R-loops under certain circumstances.
They also learned that, while E. coli also possess Rho, a well-studied enzyme capable of pulling R-loops apart, Rho cannot fully compensate when RapA is absent. “When you knock out RapA, Rho has to work overtime,” Brewer says. “It appears that RapA and Rho work as complementary—not redundant—safeguards for genome stability when E. coli are subject to high-salt stress.”
The implications could be vast. Darst, Brewer, and colleagues suspect that RapA—or something like it (an RNAP release factor, so to speak)—exists not only in E. coli but in all bacteria and, perhaps, in all cells. Uncovering similar mechanisms in other organisms could inspire new strategies to target diseases linked to transcription-related genome instability.
“We predict that other enzymes likely serve similar functions across the tree of life,” says Darst. “The more we learn about these mechanisms, the more we deepen our understanding of how cells safeguard their genomes.”



