Scientists finally know how cells build a structure that lets them migrate. What they learned could help treat cancer
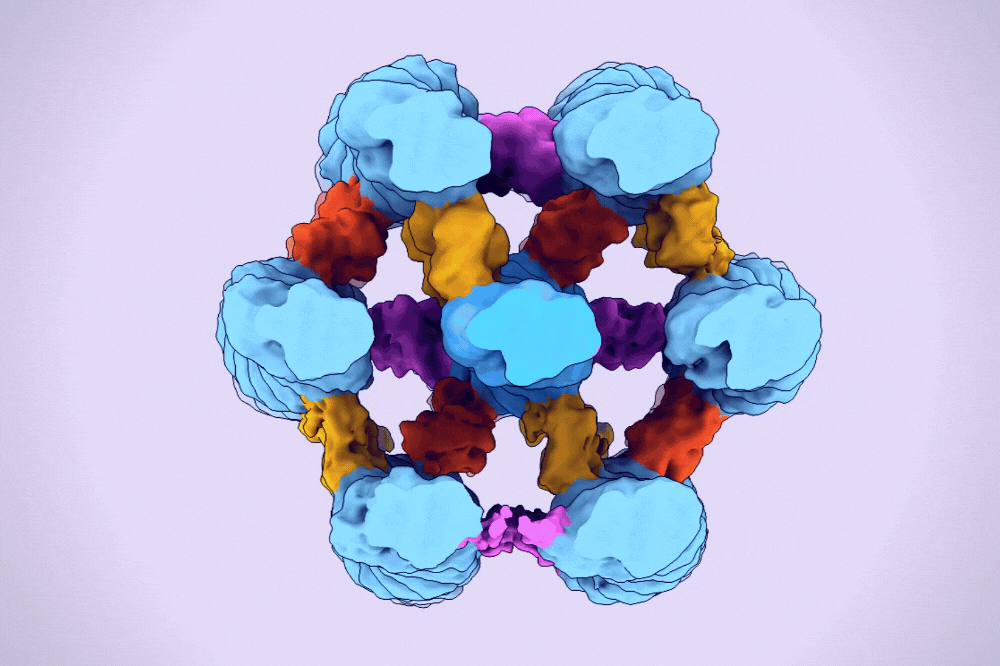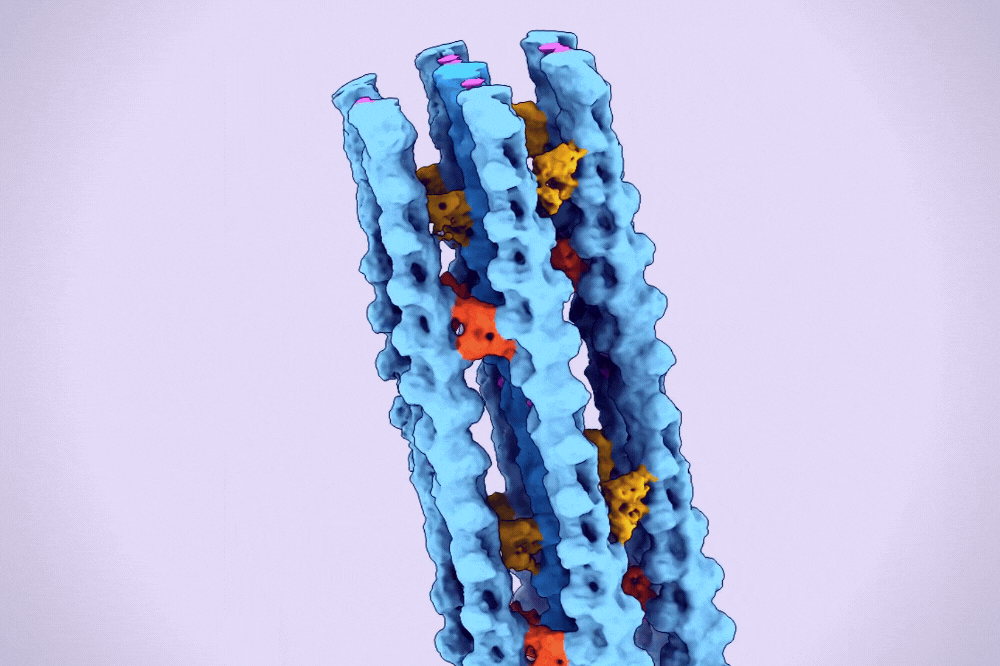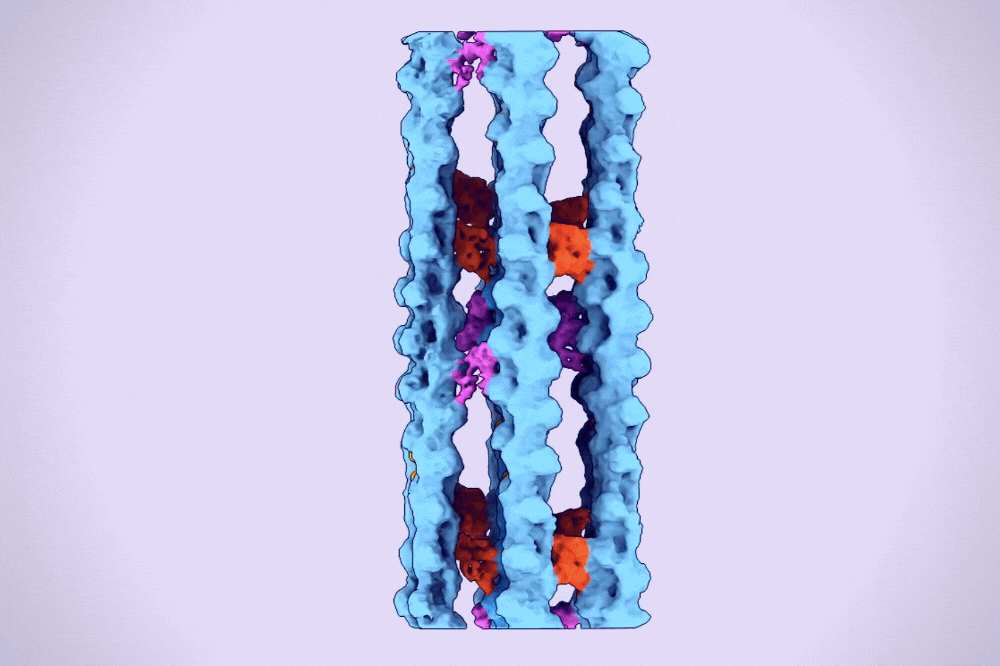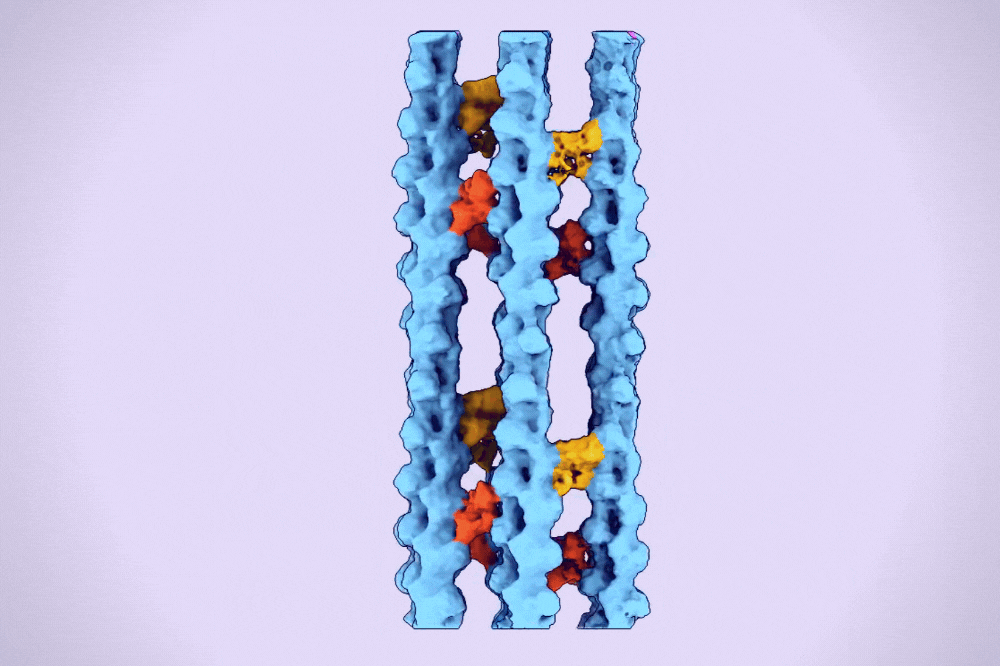
A hexagonal bundle of actin filaments linked together by fascin proteins. (Alushin Lab)
Some of the body’s cells stay put for life, while others are free to roam. To move, these migratory cells rely on filopodia—sensitive, finger-like protrusions that reach out from the cell membrane into the local environment. In a healthy cell, this can be a lifesaver: say, when an immune cell is speeding to the site of an infection. But filopodia can also wreak havoc: metastatic cancer cells use them to invade new regions of the body.
Filopodia are composed of hexagonal bundles of proteins that give them structure and strength. How these intricate bundles come together has been a puzzle for more than 40 years. A major piece of that puzzle has now been solved by Rockefeller University’s Laboratory of Structural Biophysics and Mechanobiology(opens in new window), which developed advanced imaging technology to reveal how underlying proteins build these cohesive assemblies.
The findings(opens in new window), published in Nature Structural & Molecular Biology, may improve some cancer treatments already in development, says first author Rui Gong, a research associate in the lab. “Understanding the structure of filopodia and the changes they undergo may help to refine these therapies or inspire new ones,” he says.
Where else this discovery leads remains to be seen. The study marks the first time such a complex higher-order protein assembly has been imaged at the atomic level—a technological advance that other scientists can now use to study similarly complex configurations. “Until now, it hasn’t really been possible to visualize their internal structure in any significant detail,” says lab head Gregory M. Alushin. “Going forward, hopefully we’ve made it easier to study these protein networks, where function emerges at the level of thousands of molecules.”
The forces at work
Alushin’s lab specializes in understanding the cytoskeleton—the network of protein filaments, including actin, that form a cell’s infrastructure. Actin serves many functions: it provides cells with an overall shape; helps them to generate and detect forces in their environments; facilitates the formation of axonal connections between cells; and enables cellular movement via filopodia.
These dynamic protein strands bend and flex, criss-cross each other, and even engage in tugs of war. But they only work collectively. A single actin filament is useless on its own.
“It’s like a floppy noodle,” Alushin says. “It’s not very strong, and it can’t do anything. Actin filaments have to be gathered into higher-order assemblies such as bundles to carry out any useful job.”
One type of higher-order assembly is the hexagonal bundle found within filopodia. A protein called fascin binds and bridges pairs of actin filaments, stitching them into bundles. These bundles are then encased in long membrane tubes to form filopodia, which must be strong enough to protrude beyond the cell and yet malleable enough to sweep the environment.
“They hit a sweet spot between strength and flexibility,” Alushin says.
How fascins manage this assembly has been a “known unknown” for decades. In the 1970s, scientists tried to re-create hexagonal bundles by using wooden dowels representing actin filaments with small bits of wood representing fascin-like bridges interspersed between them. It was impossible to create a bundle without distorting the ersatz fascin.
A better view
More recently, high-imaging technologies such as cryo-EM and tomography enabled the first images of these bundles, but they were only blurry glimpses. For the current study, the researchers, co-led by Gong and former Rockefeller graduate student Matthew Reynolds, significantly improved upon an computational image analysis approach they developed in 2022(opens in new window) that involves “denoising” the images.
The result was the first clear three-dimensional images of fascin proteins as they bridged actin filaments.
“We saw real bundles composed of thousands of fascin molecules and hundreds of actin filaments, and we were able to map their spatial positioning,” Gong says. “We saw how the structure of fascin gives rise to its function as an actin bundler and figured out the detailed chemistry of its actin binding sites.”
One of the most surprising findings was that fascin is quite improvisational. There are many ways for the protein to build a bundle.
Fascin may have evolved this skill because of the questionable construction materials it has to work with. “Because actin filaments are like twisty ribbons, they’re not great for building a firm hexagonal structure like you find in filopodia,” Gong notes.
To overcome this problem, fascin has a structural flexibility that allows it to slip in between the filaments in a variety of places and fold itself into the shape needed to link them together.
“A fascin protein can accommodate all kinds of imperfections. It acts like a molecular hinge that can hold a number of intermediary positions between open and closed. It can also rotate its position for a better fit,” Alushin says. “Despite being a small and ostensibly simple protein, it has very complicated physical behaviors.”
Stopping filopodia in their tracks
Fascin dysregulation is a clinical biomarker for metastatic cancer. In migratory cells, an overabundance of fascin leads to a filopodia building frenzy, which can accelerate metastasis. And stationary cells with too much fascin gain an abnormal—and dangerous—ability to move.
“When this overexpression happens in cells that should be locked into place, such as epithelial cells, they can build filopodia, which they’re not supposed to have,” Alushin says. “Then they can crawl away from their neighbors and in the process abandon their regular cellular functions.”
Their findings may help improve the design and effectiveness of fascin inhibitors, which are currently in clinical trials, Gong adds. These inhibitors aim to halt metastasis by preventing fascin from binding actin filaments and gathering them into bundles within filopodia. Immobilized, the cancer cells are stopped in their tracks.
It was thought that the inhibitors work by blocking fascin’s actin binding sites, but the Rockefeller researchers discovered that instead, they prevent fascin from undergoing the shape changes needed to fit in its binding location—a new understanding that the team hopes could translate into clinical applications.
“We’ve been able to detail essential design principles for the bundles, which could be really helpful information for finding new ways to interfere with their construction,” Alushin says.



