Rockefeller University Cell Biologist, Günter Blobel, Wins 1999 Nobel Prize in Medicine

Günter Blobel, M.D., Ph.D
Rockefeller University cell biologist Günter Blobel, M.D., Ph.D., was awarded the 1999 Nobel Prize in Physiology or Medicine today. Blobel, the John D. Rockefeller Jr. Professor at The Rockefeller University and a Howard Hughes Medical Institute investigator, heads the Laboratory of Cell Biology. Günter Blobel, M.D., Ph.D.
Blobel studies the process by which newly made proteins are transported across the membranes of cell structures called organelles. Because the accurate distribution of proteins to their proper places in the cell is necessary for a cell to function, these findings have an immediate bearing on many diseases, including cystic fibrosis, Alzheimer’s disease and AIDS.
An average cell possesses about a billion protein molecules that exist in thousands of types and constantly need replacement. Making proteins and shipping them to appropriate destinations, such as the cell’s internal organelles, is a vital activity in cells. Proteins are manufactured by cellular structures called ribosomes. Pioneering research by Blobel and his associates revealed how proteins are transported from ribosomes and integrated into other organelles or transported out of the cell.
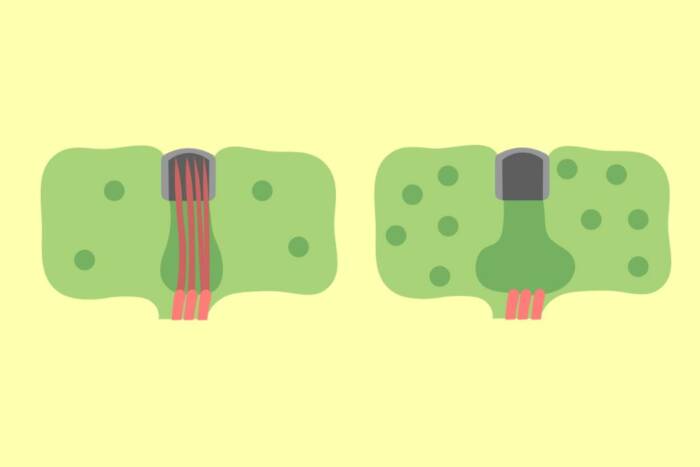
Work in Blobel’s laboratory revealed the existence of a zip code system in the cell. Each newly made protein has an organelle-specific address, a stretch of the protein referred to as a signal sequence that is recognized by receptors on an organelle’s surface. Blobel and his colleagues also showed that, for at least one organelle called the endoplasmic reticulum, the binding of the signal sequence to its receptor opens a watery channel in the membrane through which the protein can travel. Blobel now works on identifying similar channels in other organelles.

Blobel (center) and members of his lab at Rockefeller University.
Current research in Blobel’s laboratory also explores the movement of proteins across nuclear pore complexes (NPCs), huge protein units suspended in the circular openings within the membrane of a cell’s nucleus. NPCs can accommodate the passage of large molecular assemblies, such as RNA or DNA bound to proteins. Each NPC mediates as many as 10 import and 10 export events per second. His laboratory recently determined the three-dimensional structure of a complex of transport factors called karyopherin-beta 2, which binds to proteins and targets them to the nuclear pore complex. Blobel (center) and members of his lab at Rockefeller University.
Click here to see an animated model of his research:http://www.rockefeller.edu/pubinfo/proteintarget.html