Cells Leap Frog On The Way To Becoming Nerve Cells
Clues to movement may help design therapies for diseased, injured brains.
Kids aren’t the only ones who play Leap Frog. Cells destined to become nerves in the brain do too, according to scientists from The Rockefeller University, who published their findings in the Feb. 16 Science. The information reveals a new mechanism of migration used by nerve cell precursors and could help in the design of therapies that would replace diseased or injured nerve cells in the human brain.
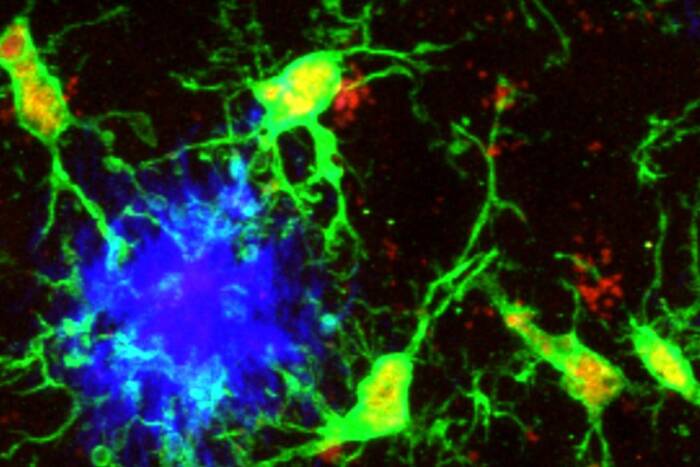
 The study is the first to show that neuroblasts, cells that mature into nerve cells, migrate within the brains of adult mice by following each other and not by adhering to the elongated processes of guide cells. The neuroblasts move to find their site of differentiation and mature into specific kinds of nerve cells.
The study is the first to show that neuroblasts, cells that mature into nerve cells, migrate within the brains of adult mice by following each other and not by adhering to the elongated processes of guide cells. The neuroblasts move to find their site of differentiation and mature into specific kinds of nerve cells.
“The neuroblasts literally crawl over each other as they move through a tunnel formed by the brain’s glial cells,” explains Arturo Alvarez-Buylla, Ph.D., associate professor and head of the Laboratory of Neurogenesis. “Previous studies showed that neuroblasts in developing brains and adult birds move by climbing on glial cells. Instead of attaching to glial cells or parts of nerves, we found that neuroblasts in adult mice follow each other to reach distant parts of the brain where they mature into nerve cells.”
“By learning how the migration is regulated, we may be able to develop ways to steer the cells to specific locations in the adult brain,” explains Alvarez-Buylla. “Such research could lead to the development of therapies for Alzheimer’s disease, Parkinson’s disease or damage from injuries.”
The neuroblasts move at high speeds for cells, about 30 microns–less than two thousandths of an inch–in an hour, through complex tissue between two regions of the brain separated by several millimeters. In mammals and birds, neuroblasts are born in parts of the brain known as the ventricular zone and the subventricular zone. The zones are the tissues that surround the cavities in the brain.
In the current research, Alvarez-Buylla and his colleagues noted adult mouse neuroblasts use chain migration to negotiate movement for long distances in a cell, up to 5 mm–almost one-quarter inch, from the subventricular zone to the olfactory bulb, where smelling functions are based. After arriving in the bulb’s core, the cells separate and move to the bulb’s outer layers and mature into nerve cells.
Images from electron micrographs showed that glial cells used their bodies and branch-like processes to form a tube-like structure around the migrating cells. The researchers propose that the tubes of glial cells may provide directional cues to the olfactory bulb or also may restrict the neuroblasts from moving into other regions of the brain.
Alvarez-Buylla’s coauthors include Carlos Lois, Ph.D., of Rockefeller and Jose-Manuel Garcia-Verdugo, Ph.D., of the University Valencia in Spain. The National Institute of Neurological Disorders and Stroke and the National Institute of Child Health and Human Development supported the study.