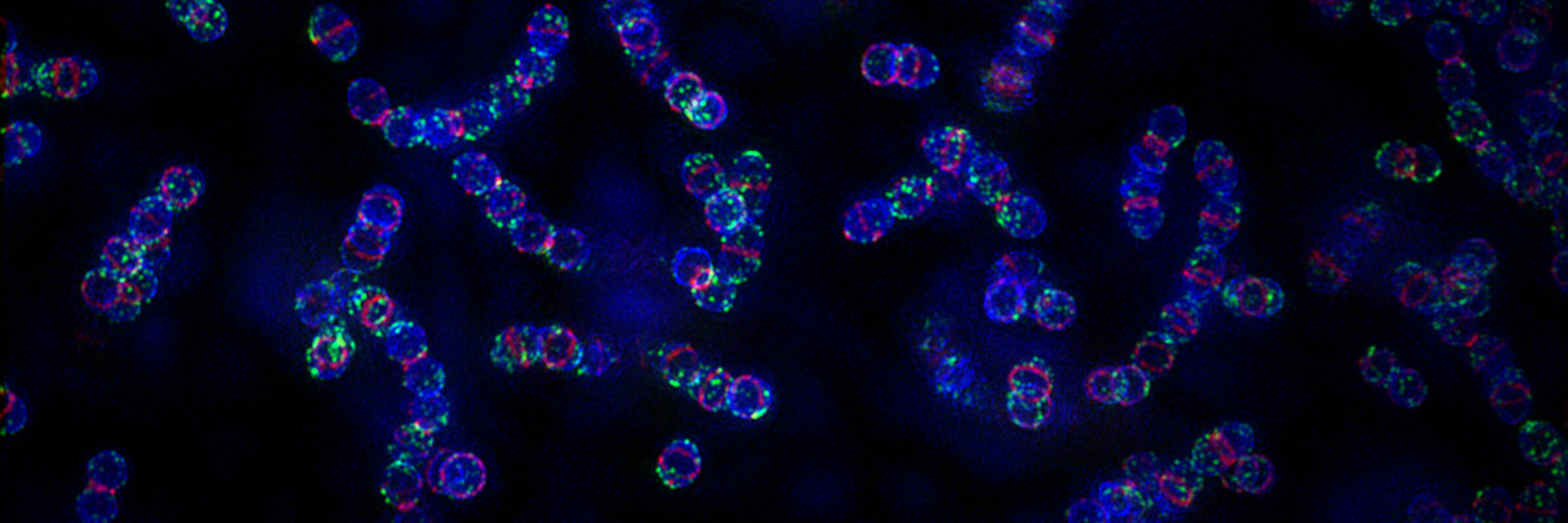
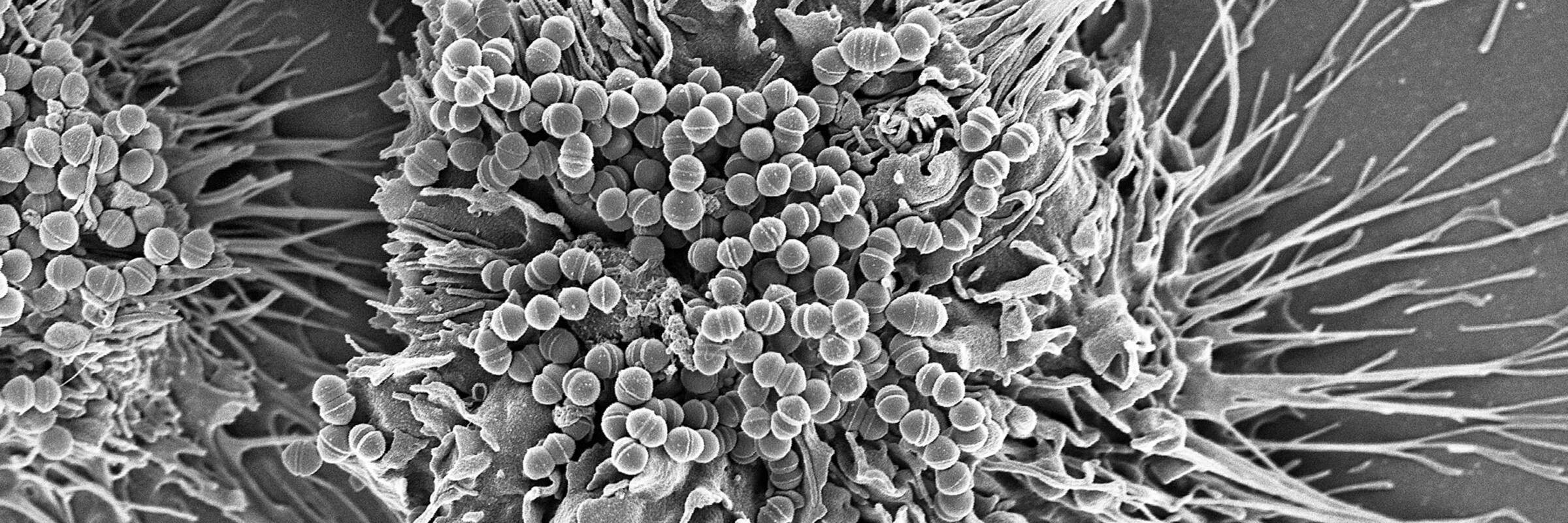
Antibiotic resistance is a critical global health threat driven by the overuse of antibiotics in healthcare, agriculture, and farming. Resistant strains like MRSA and multi-drug-resistant E. coli render treatments ineffective, leading to prolonged illness, higher costs, and increased mortality. The spread of resistance genes and slow antibiotic development worsen the crisis, threatening medical progress and requiring urgent action in research, policy, and education.
Our lab has risen to this challenge by developing one of the most promising alternatives to traditional antibiotics: phage lysins. Phage lysins are enzymes produced by bacteriophages (viruses that infect bacteria) to release viral progeny from infected bacterial cells. When applied externally to susceptible bacteria, purified forms of these enzymes cleave bonds in the bacterial cell wall, causing rapid lysis through osmotic disruption. Due to their critical role in phage survival, lysins have evolved to target essential bacterial cell wall bonds that are difficult for bacteria to alter. Remarkably, no resistance to lysins has been observed in over 20 years of development.
Over the years, our lab has developed lysins effective against nearly all major gram-positive and many gram-negative human pathogens. Notably, one of our lysins targeting methicillin-resistant Staphylococcus aureus (MRSA) has been licensed and successfully completed phase 1 and phase 2 human clinical trials for the treatment of MRSA bacteremia and endocarditis—making it the only antibiotic alternative to achieve these FDA milestones.
Building on this success, the lab’s focus is to harness gram-positive and gram-negative lysins for combating bacterial diseases in both acute and chronic infections, as well as for prophylactic applications. Unlike antibiotics, which frequently encounter resistance, lysins have shown no resistance to date, opening possibilities for their use in scenarios where antibiotics have proven ineffective or inappropriate.