Lulu and Anthony Wang Laboratory of Neural Circuits and Behavior

Vice President for Academic Affairs


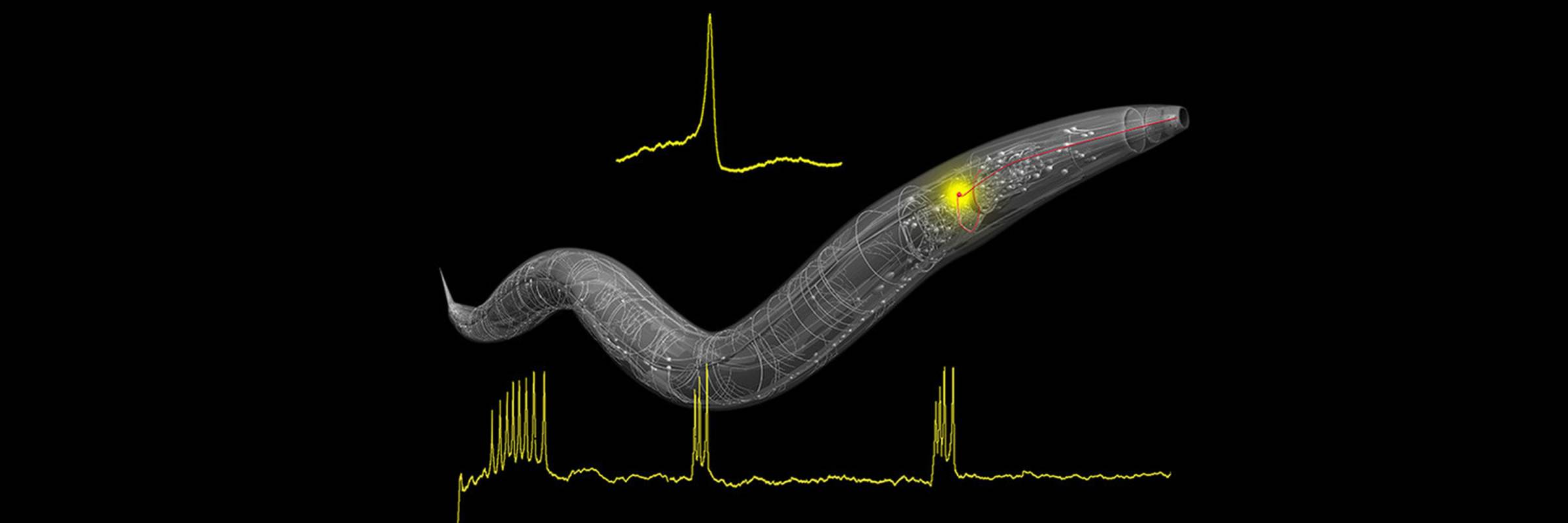
How do genes and the environment interact to generate a variety of behaviors? How are behavioral decisions modified by context and experience? The Bargmann lab is studying the relationships between genes, experience, and behavior in the nematode C. elegans, whose nervous system consists of only 302 neurons with reproducible functions, morphologies, and synaptic connections. Despite this simplicity, many of the genes and signaling mechanisms used in the nematode nervous system are similar to those of mammals. The ability to manipulate the activity of individual genes and neurons in C. elegans makes it possible to determine how neural circuits function and change over time.
C. elegans has a rich olfactory sense; it detects hundreds of different odors and pheromones, discriminates among them, and generates a variety of responses to the odor cues. Each sensory neuron has dynamic responses that reflect both the identity and the history of the odor, enabling it to perform a set of computations that are stereotyped in a single neuron, but differ among neurons. The sensory neurons synapse onto integrating neurons, which also use stereotyped cell type-specific computations to combine sensory inputs. Finally, groups of integrating neurons coordinate their activity to generate reliable systems-level dynamics coupled to behavior. The lab characterizes the effects of specific molecules, neurons, and circuits across these levels of integration, asking what functional properties arise at each level of analysis.
In addition to the fast processing of sensory information by fast neurotransmitters, behavior is reversibly modified over longer timescales by neuromodulators such as serotonin, dopamine, and neuropeptides. Neuromodulators are highly conserved in evolution, and implicated in human as well as animal motivational and emotional states. The lab has identified roles for neuromodulators in spontaneous behaviors, learned behaviors, and age-specific behaviors of C. elegans, and determined the mechanisms by which they act. Their results indicate that wireless, distributed neuromodulatory signals rapidly rewire the functional properties of the fixed C. elegans nervous system, dynamically changing the flow of information across synapses and circuits. Neuromodulatory systems also provide a substrate for genetic variation in behavior within and between species. Current experiments explore how neuromodulatory signals are coupled to context and behavioral states, and how they affect the flow of information between neurons at different timescales.
Bargmann is a faculty member in the David Rockefeller Graduate Program, and the Tri-Institutional M.D.-Ph.D. Program.