Completed Projects
Updated December 12, 2023
In the Vosshall Laboratory, PhD students and postdoctoral fellows carry out their own highly original projects. Below is a partial reverse chronological list of people and projects from 2000 to the present
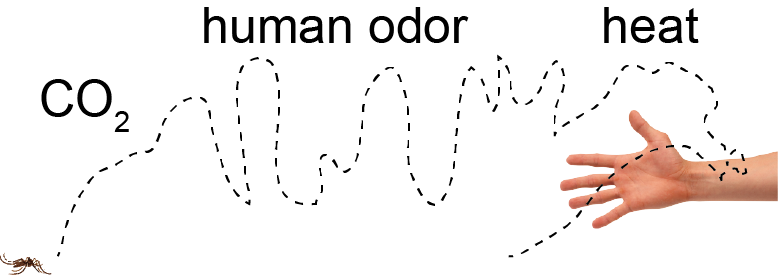
Sustained motivation during mosquito host seeking – Trevor Sorrells PhD (2016-2022)
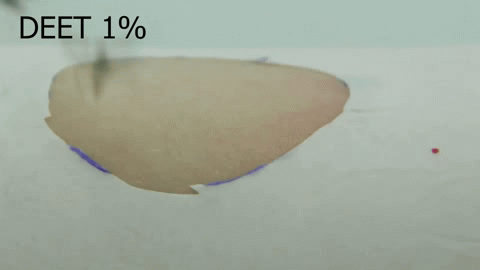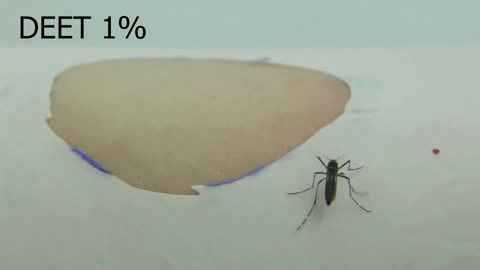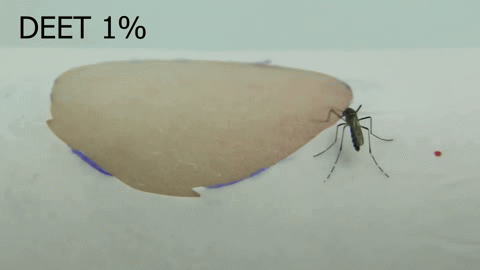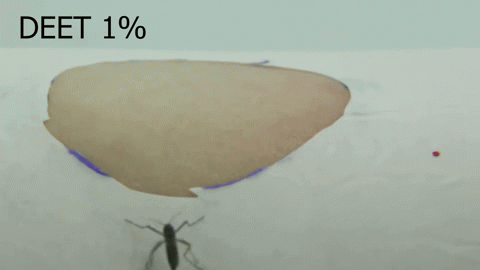
Chemosensory coding of DEET in Aedes aegypti tarsi – Olivia Goldman (2018-2023)
An orco mutant female mosquito avoiding DEET-treated skin.
The insect repellent DEET is a synthetic compound, discovered in 1946 by the USDA as part of a large chemical screen. Since its discovery, DEET has become the most broadly used and effective arthropod repellent available, but the details of how DEET works remain elusive. We discovered that, in addition to being able to smell DEET, Aedes aegypti can detect DEET upon contact via their legs (Dennis et al, 2019). Using a combination of imaging techniques, molecular profiling, and genetic manipulation, we are further investigating the molecular mechanisms of DEET contact detection in the mosquito leg.
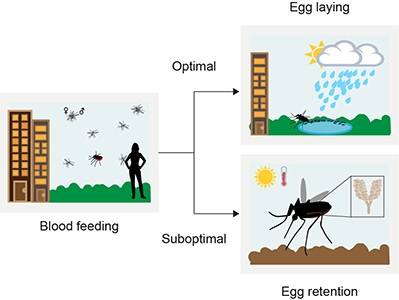
Drought resilience and reproductive capacity in Aedes aegypti – Krithika Venkataraman (2015-2022)
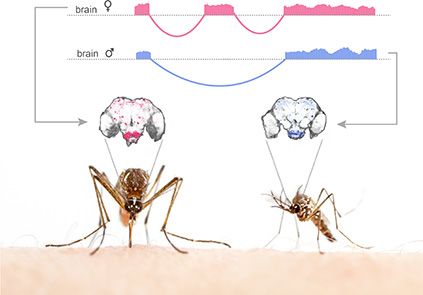
Genes and Neural Circuits underlying Sexual Dimorphism in Mosquito Host-Seeking Behavior – Nipun Basrur (2016-2021)
Sex-specific splicing of a representative gene and its expression pattern in male and female mosquito brains
A female mosquito is expertly adapted to find a vertebrate host to take a blood-meal, which she requires to develop her eggs. Only females carry out this specialized innate behavior. Since host-seeking and blood-feeding are sexually dimorphic behaviors, the underlying genes and neural circuits controlling them are likely also sexually dimorphic. I hypothesize that sex-specific splicing of key genes controls sexual dimorphism in host-seeking behavior. To test this, I have identified genes that are sex-specifically spliced in mosquito brains, and I am generating mutants that disrupt the sex-specific isoforms of each gene, and developing transgenic reagents to visualize the cells expressing these genes.
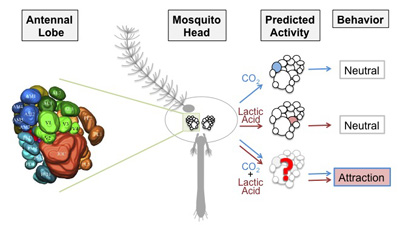
Processing Human Cues in the Mosquito Brain – Meg Younger PhD (2014-2021)
Models of multi-sensory integration in the antennal lobe of the female mosquito
Female mosquitoes require a blood-meal for reproduction, and show intense attraction to human hosts. They rely on host sensory cues, including carbon dioxide (CO2) in breath, and components of human body odor, such as lactic acid. These stimuli alone elicit little or no attraction, but in combination they synergize to trigger host-seeking behavior.
It is unknown where and how any human host cues are represented in the mosquito brain. It is also unknown how human host cues synergize to drive host attraction and ultimately trigger biting behavior. To address these questions I am using two-photon excitation microscopy and electrophysiology to measure activity in the mosquito central nervous system. I am focusing on the antennal lobe of the brain, a region that is an important site of odor processing in other insects.
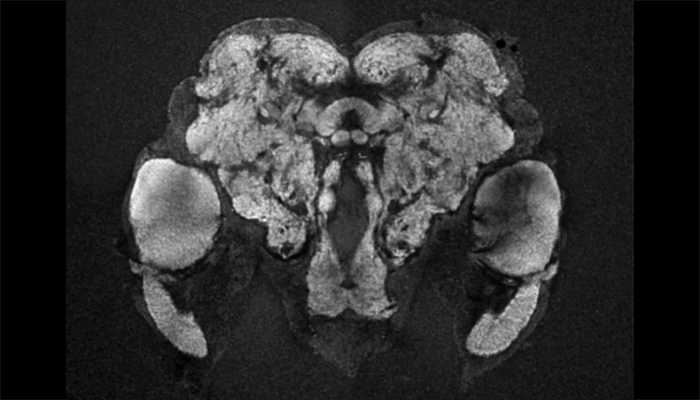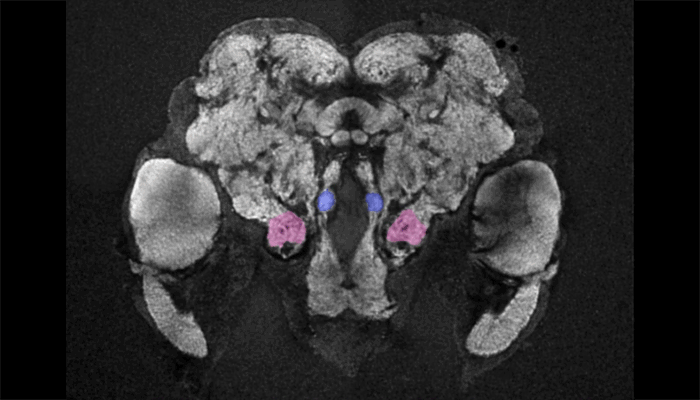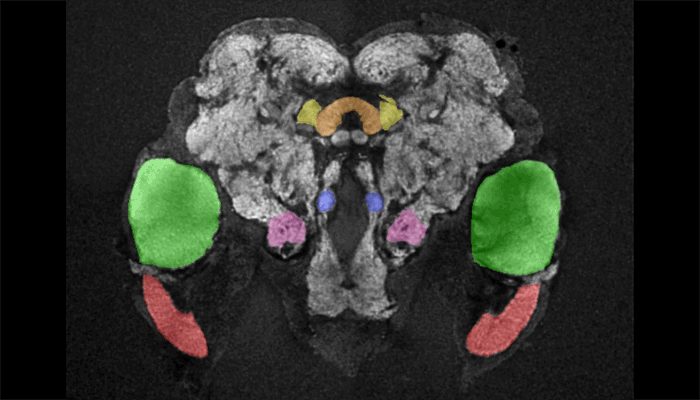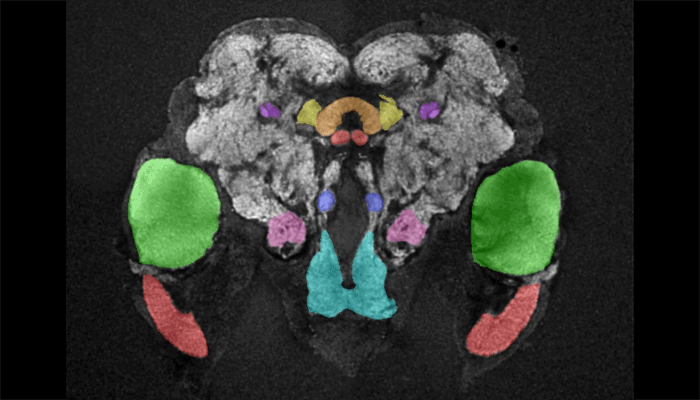
mosquitobrains.org: An Online Atlas of the Brain of Aedes aegypti – Meg Younger PhD (2014-2021)
Female mosquito brain
As a tool to understand the neural circuitry that underlies mosquito behavior, we have generated a reference brain for Aedes aegypti. This is the brain from a female mosquito that has different brain regions annotated for viewing in an online browser. 3D-reconstructions are also available for viewing. Brains imaged on a confocal microscope can be registered onto the female reference brain with a free and open source software pipeline, which is available at mosquitobrains.org. This will allow users to examine how expression patterns of new transgenic lines intersect. Lastly, this website will serve as a repository for mosquito neuroanatomy data, as new transgenic reagents are generated in this rapidly growing field.
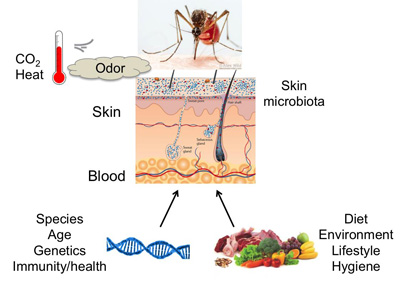
The role of host physiology in mosquito attraction – Maria Elena De Obaldia PhD (2014-2021)
Model of physiological factors that may contribute to mosquito attraction to vertebrate hosts.
Unlike most insect species, which feed on plants, female mosquitoes require a vertebrate blood meal to produce each batch of eggs. This mosquito blood-feeding behavior facilitates disease transmission among populations because females feed on multiple humans throughout their lifetime. There is intense interest in developing interventions to diminish mosquito attraction to humans, thus limiting the spread of mosquito-borne disease.
Mosquitoes use multimodal sensory cues to locate human hosts in their environment, including heat, CO2, and human odor. Differences in skin odor alone can modulate mosquito attraction, when temperature and CO2 are held constant. Physiological factors that contribute to odors emanating from human skin are unclear, but may include: genetic factors, diet, immunity, blood metabolites, and the composition of skin microbiome. I will investigate the contribution of physiological factors to human odor and mosquito attraction using mice as model vertebrate hosts. These studies aim to identify mechanisms of mosquito host preference.
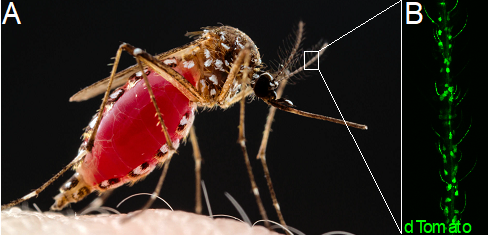
Encoding and modulation of Aedes aegypti mosquito host-seeking behavior by internal state – Margaret Herre (2016-2021)
(A) A female Ae. aegypti mosquito engorges on a blood-meal from a human host. (B) The QF2-QUAS binary expression system drives expression of dTomato and GCaMP6s in olfactory sensory neurons in one of the mosquito olfactory organs, the antenna.
For my main thesis research, I am studying the role of the steroid hormone 20-hydroxyecdysone (20E) in regulation of mosquito host-seeking behavior. I have established that 20E suppresses host-seeking drive without affecting other feeding behaviors and have shown that receptor for 20E, the ecdysone receptor (EcR) is expressed in olfactory sensory neurons. I hypothesize that 20E is acting directly at the level of the sensory neuron to affect how female mosquitoes perceive human odor. EcR is a nuclear hormone receptor that is required for development. Since we currently are unable to generate a conditional EcR knockout mosquito, I am taking orthogonal approaches to determine the role of EcR and 20E signaling in sensory neurons. These experiments include determining the transcriptional signature of 20E signaling in olfactory organs with RNA-seq and labeling and manipulating EcR chemosensory neurons using the split QF2-QUAS binary expression system.
In collaboration with Meg Younger, a postdoc in our lab, I seek to understand how the mosquito olfactory system is organized. A fundamental molecular feature of olfactory systems is that individual neurons express only one olfactory receptor in each olfactory sensory neuron. In both mice and Drosophila, neurons that express the same olfactory receptor then converge in the same regions in the primary olfactory processing centers in the brain. This organization enables olfactory systems remarkable specificity. We made the surprising discovery that in Aedes aegypti, olfactory sensory neurons express multiple classes of olfactory receptors in each neuron. Our ongoing experiments are determining the functional significance at the level of the sensory neuron, as well as behavioral consequences for discriminating attractive and aversive odorants.
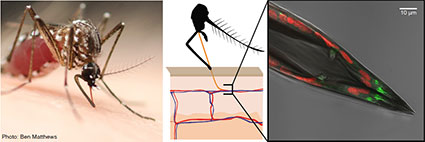
A Taste of Blood – Veronica Jové (2015-2020)
When a female mosquito takes a blood meal from a human host, she uses the dedicated blood-feeding appendage, the stylet, to pierce skin and pump blood. The needle-like stylet contains female-specific cells and neuronal processess that may be used to detect blood
A female mosquito must take a blood meal from a vertebrate host to produce eggs, and in doing so she transmits diseases such as Zika. The mechanism by which a female detects blood and initiates this robust behavioral program is unknown. Early studies have demonstrated that protein is not sufficient or required to initiate blood-feeding, although it is essential for egg production. Surprisingly, ATP in saline is sufficient to initiate blood-feeding in the absence of blood components and chemosensory cues from skin. We hypothesize that the decision to engorge on blood is mediated by the rapid sensory detection of ATP as a proxy for blood. To elucidate how the detection of ATP leads to the initiation of blood-feeding behavior, we are focusing on the dedicated blood-feeding appendage, the stylet, which is the only innervated appendage that directly contacts blood. We are using behavior, genomics, and calcium imaging to identify the cells and receptors that detect ATP. We will use this information to map the circuitry that detects blood and regulates a behavior responsible for transmission of vector-borne diseases to millions of people across the globe.
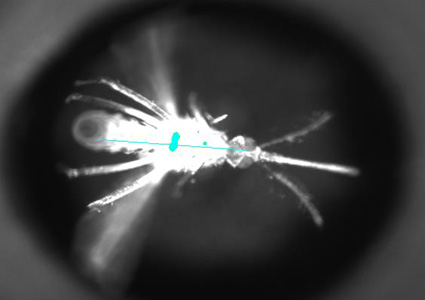
A tethered mosquito orienting towards a dark stripe
Female mosquitoes require vision to obtain a blood-meal from vertebrate hosts. Without visual feedback, mosquitoes are unable to track hosts, and visual cues may form an important part of a mosquito’s percept of a host. Yet we know neither what specific visual cues host-seeking mosquitoes attend to, nor how these visual inputs interact with other sensory modalities. We are characterizing visual contributions to host-seeking in female Aedes aegypti. We are defining how Aedes aegypti mosquitoes use vision to find hosts using both tethered and free-flying behavioral assays. We are also establishing a platform for studying how these relevant visual stimuli are represented in the mosquito brain. Results will not only illuminate the behavior of a globally important disease vector, but also give insights into how visual inputs interact with other sensory inputs in a robust, ethological context.
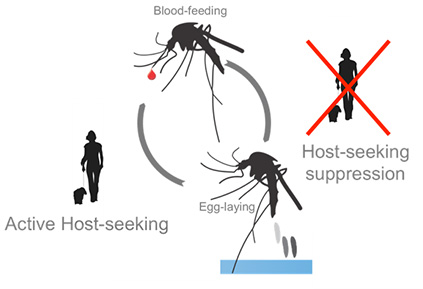
Cyclical suppression of mosquito host-seeking behavior by a blood meal
Female mosquitoes are attracted to humans by a multitude of sensory cues emitted from hosts, including heat, carbon dioxide (CO2), and body odor. The strong drive to seek a human host for a blood meal is only expressed when the female needs to produce and lay eggs—a process known as oviposition—when protein from a blood meal is required to produce yolk and mature eggs. Previous work suggests that neuropeptide signaling is involved in reprogramming female behavior from host-seeking to oviposition. Previous studies suggest that suppression of host-seeking behavior has been associated with Neuropeptide Y related signaling and our work implicates a specific NPY-like receptor (NPYLR7). These findings suggest that neuropeptides may modulate sensory circuits, including those involved in smell, taste, and CO2 perception, rather than directly inhibit host-seeking behavior itself. This makes neuropeptide signaling a promising candidate for mediating the behavioral switches that occur during mosquito reproductive cycles. The goal of this project is to identify neuropeptide signaling pathways that control the cyclical regulation of host-seeking behavior in female Aedes aegypti mosquitoes.
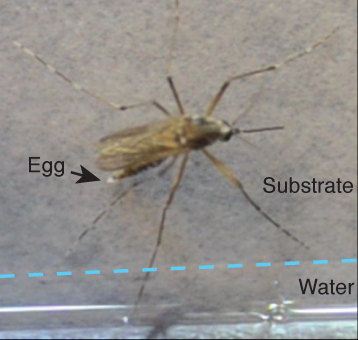
A female Aedes aegypti mosquito laying an egg immediately above the water line of a pool of standing water
After taking a blood meal, female mosquitoes undergo a radical behavioral shift. Instead of searching for vertebrate hosts, they instead seek an appropriate aquatic location to lay eggs on a rough surface just above the water line, a behavior known as oviposition. This reproductive behavior involves three critical steps: orienting towards water at long range using cues such as humidity, the detection of standing water, and the evaluation of water osmolarity and quality. We have developed several detailed behavioral assays to investigate mosquito oviposition behavior, and begun a comprehensive analysis of gene expression in sensory tissues of Aedes aegypti with the goal of identifying and mutagenizing genes involved in oviposition and oviposition site selection.
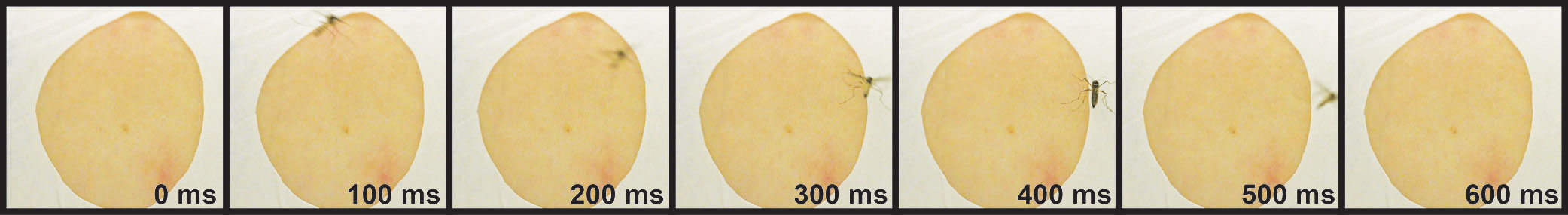
DEET contact chemorepellency: orco mutant mosquito lands on a DEET-treated arm at 400ms, but does not blood feed
The insect repellent DEET is a synthetic compound, discovered in 1946 by the USDA as part of a large chemical screen. Since its discovery, DEET has become the most broadly used and effective arthropod repellent available, but the details of how DEET works remain elusive. We discovered that Aedes aegypti orco mutants cannot smell DEET but will not bite DEET-treated arms. We have started to characterize this behavior using quantitative behavioral analysis and manipulation. Next, we are working towards discovering the molecules mosquitoes use to sense DEET on contact, and confirming these candidates using targeted mutagenesis.
According to the National Health and Nutrition Examination Survey, 14.1 million American citizens suffer from smell impairment and 3.6 million have completely lost their sense of smell, which is a condition named anosmia. Even though smell disorders can present insidiously, dramatic consequences on quality of life, mental well-being, and mortality occur. The management of these conditions urgently needs improvement. We explore the possibilities of addressing current limitations in smell tests to develop ground-breaking and innovative tools to measure a patient’s sense of smell. Although smells in nature are often composed of multiple molecules (e.g the smell of roses contains more than 200 molecules), current smell tests are based on single volatile molecules such as n-butanol or phenylethyl alcohol. This constitutes two large problems. First, due to large genetic variability in odorant receptor genes among the population, individuals have variable sensitivity and perception to a given molecule. Second, the results of currently used smell tests depend on a patient’s cultural background and previous experiences with these molecules. These two issues limit the generalizability and accuracy of these tests. We use complex mixtures of molecules to overcome these limitations and to develop an “olfactory resolution test.” The goal of this work is to develop high performance clinical tests to improve the management of smell disorders for clinicians from around the world.

A thermal image of Roman Corfas
Human body heat is a strong host-seeking cue to a female mosquito searching for a blood meal. In fact, female Aedes aegypti are attracted to warm inanimate objects, and probe at them as though they were a host. We have established quantitative assays to measure heat-seeking behavior in Aedes aegypti, as well as to test interactions between warmth and other host-seeking cues such as carbon dioxide. Using these assays, along with targeted mutagenesis, we are examining the molecular basis of thermosensation and heat-seeking behavior in the mosquito.

The EXPRESSO assay: automated fly capillary feeding assay
The perception of hunger and satiety and the regulation of food intake are controlled in the nervous system by evaluating internal metabolic state and external chemosensory information. The proper function of this complex physiological system is essential for humans and other animals to balance nutrient intake, to maintain stable body weight, and to regulate metabolism. After fasting, the drive to eat increases, leading to enhanced foraging and food intake until the energy deficiency is compensated and metabolic homeostasis returns. When the balance between hunger and satiety is perturbed, food intake is misregulated, leading to excessive or insufficient eating. In humans, this abnormal nutrient consumption cause metabolic conditions, such as obesity, and eating disorders, such as anorexia nervosa, bulimia, and binge eating disorder. Similar to other animals and humans, feeding state in the fly (Drosophila melanogaster) regulates attractiveness to food odors and responsiveness to sugars. After fasting, flies increase their food intake to compensate for the energy deficiency incurred. In this project, we aim to discover novel genes that are essential for proper food intake in a model organism, Drosophila melanogaster, in which large behavioral genetic screens are feasible. Our long-term goal is to relate our results to the homologous mammalian genes with similar functions and thus to discover conserved pathways that regulate hunger and satiety. We are also developing technologies such as an automated instrument called EXPRESSO that measures real-time food consumption, which will greatly accelerate the feeding research in the fly.

Olfactory stimuli are not well understood. Olfactory psychophysics has traditionally relied on testing the perception of small groups of odors of special interest. It has been difficult to draw general conclusions about olfactory psychophysics from these vignettes. We therefore decided on an unbiased, high-throughput approach in which we test the perception of large numbers of olfactory stimuli. One current study addresses the connection between physical and chemical properties of odor molecules and their perceived odor quality. Our most recent study is designed to answer the question how many distinguishable odors there are.
As part of our overall mission to understand the genetic and neuronal basis of how odors guide behaviors, we are conducting psychophysical studies with human subjects to find correlations between genetic variability in odorant receptor genes and variability in cognitive and physiological responses to odors. In collaboration with Hiroaki Matsunami’s group at Duke University we have shown that variability in one odorant receptor gene, OR7D4, influences the perception of odorous steroids. Currently we are extending our studies to include more odors and more odorant receptor genes. We are also interested in how variability in OR genes influences perceived odor quality. To include subliminal and physiological responses to odors in this project, we also investigate the genetic basis of changes in salivary cortisol and mood induced by an odorous steroid.

Olfactometer assay used to determine how attractive a human subject’s arm is to mosquitoes
It is a widespread observation that mosquitoes prefer some people over others.
In our well-controlled laboratory setting, we found that this is also true — mosquitoes go crazy when offered a whiff of certain human subjects, while they find the smell of other subjects only mildly compelling. Many hypotheses have been put forth, both anecdotally and scientifically, to attempt to explain these large differences in mosquito attraction, but none fully explain the cues mosquitoes use to choose between individuals.
We are currently conducting studies with human subjects from the New York City area to seek out better answers to this question. In these studies, we are determining how attractive subjects are to mosquitoes in our laboratory setting (using an olfactometer, pictured on the right) and then searching for physiological correlates of this behavior. Understanding what drives mosquitoes to target specific human hosts may eventually help us to develop new tools to reduce the spread of deadly mosquito-borne diseases.

Female Aedes aegypti mosquitoes blood-feeding from a heated membrane feeder in the presence of supplemental CO2
Carbon dioxide (CO2) exhaled in breath is a potent chemosensory cue that lures deadly mosquitoes towards humans to blood-feed. Aedes aegypti senses CO2 using a specialized class of olfactory sensory neuron on the maxillary palp that co-expresses three gustatory receptors (Gr1, Gr2 and Gr3) thought to mediate responses to this volatile gas. We are using genome-editing techniques, coupled with large-scale laboratory and semi-field based analyses of mosquito behavior to (i) dissect the molecular basis of CO2 reception in Aedes aegypti; (ii) determine the relative role of CO2 detection to mosquito host-seeking behavior; and (iii) identify sensory networks that ultimately drive mosquito attraction to humans.
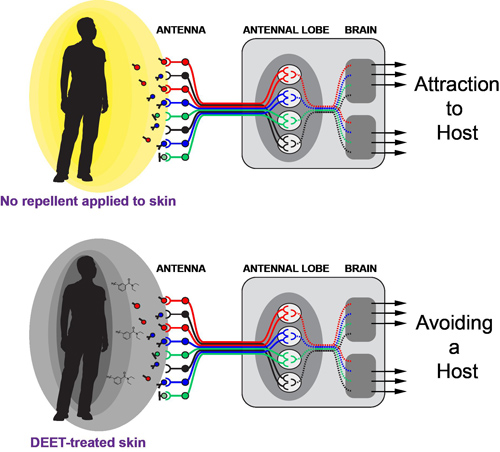
Schematic of mosquito attraction and repulsion
Host odor is a long-range, attractive cue that guides mosquitoes to their hosts. The mosquito perceives differences in host odor, both between and within species, to determine which host to feed upon. Until recently, the molecular mechanism by which mosquitoes translate host odor information into host-seeking behavior has only been inferred. To shed new light on mosquito olfaction and host-seeking behavior, we developed a technique for targeted mutagenesis in Aedes aegypti using zinc-finger nucleases. Using this technique, my co-authors and I demonstrated that orco, an olfactory co-receptor, enables host odor attraction, the preference of mosquitoes for human hosts, and DEET-driven repellency. In addition, the establishment of loss-of-function genetics in Aedes aegypti using zinc-finger nucleases has opened new paths of investigation in vector biology.
How volatile DEET drives repellency in insects is an unresolved controversy. We have identified Orco and the ORs as behaviorally relevant, olfactory receptors necessary for both Drosophila melanogaster and Aedes aegypti to avoid DEET. Experiments are underway to understand how DEET hijacks ORs to make attractive cues unappealing. We wonder whether DEET activates a specific neural circuit that drives repulsive behavior or whether DEET alters the perception of attractive odors.
We not only seek to understand how the mosquito responds to odor cues from the environment, but how the nutritional status of the mosquito regulates odor perception. The transmission of vector borne disease involves female mosquitoes making behavioral shifts from host-seeking to oviposition during each gonotrophic cycle. Understanding how these behavioral shifts are accomplished could open up new strategies for vector control. One of the advantages of Aedes aegypti is its clearly defined cyclical host-seeking behavior that is under humoral control. These behavioral changes have been correlated with alterations in antennal sensitivity to odors. To begin to address the genetic basis of these behavioral shifts, I initiated an in depth analysis of the neural and chemosensory response to blood-feeding using RNA-seq. We are in the process of identifying candidate genes that regulate the behavioral switch from host-seeking to oviposition.
My research aims to (1) identify the olfactory receptors that mediate human odor perception (2) understand the mechanism of action of DEET and (3) determine how host-seeking is regulated by changes in nutritional status during the gonotrophic cycle.
Ancestral populations of the Dengue-Fever mosquito Aedes aegypti from sub-Saharan Africa are largely generalists. They are not particularly attracted to humans, they feed on a wide variety of animals, and they breed in tree holes in natural habitats.
Outside of Africa, however, recently evolved populations of Aedes aegypti specialize on humans. They are strongly attracted to human scent, they thrive on human blood, and they breed in artificial containers in human-disturbed habitats – often even in water stored inside people’s homes. Previous work has shown that these two ecologically divergent forms of the mosquito coexist in several villages along the coast of East Africa where they maintain their differences despite living in close proximity. This project aims to (1) identify the genetic basis of recently evolved preference for human odor and (2) to describe the evolutionary processes by which this trait arose and is maintained.
This work is funded in part by a grant to Richard Axel and L.B.V. from the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative

Fly courtship behavior: A male fly (right) courting a female fly (left) while both walk across a banana slice.
How complicated behaviors arise from and are regulated by the brain is perhaps the greatest mystery in biology. Sexual behavior in the fly provides a reductionist and genetically malleable system for addressing this mystery. Drosophila melanogaster exhibit a particularly well-studied sexually dimorphic set of innate behaviors in their precopulatory courtship. In the classical view, male Drosophila initiate courtship and perform stereotyped behaviors in order to woo the female. The female, for her part, may respond by being receptive to copulation or avoiding it. We are interested in understanding how flies use courtship to make the decision whether, and with whom, to mate. The decision to mate is based first of all upon the drive to reproduce, and both the fly’s internal state and sensory information from the environment influence the choice. During courtship, flies gather information about potential mates via visual, auditory, tactile, and chemical cues. We want to understand how these cues are interpreted by the fly to decide whether or not to mate with a given individual. Previous analyses of courtship have largely focused on the behavior of the male, but given the equal importance of mating to females, we are working to understand the female’s role in the decision to mate.
Mosquito blood-feeding behavior. A female Aedes aegypti mosquito prepares to take a blood meal from a human.
Olfactory cues guide mosquitoes toward humans, from which the mosquitoes derive the blood they need to complete ovarian development. We are carrying out two conceptually related projects in mosquitoes.
In the yellow fever and dengue vector mosquito, Aedes aegypti, host-seeking is suppressed or inhibited for about 72 hours after the mosquito takes a blood meal. The molecular basis for how host-seeking behavior is regulated is unknown, but may be explained by a humoral control mechanism in which the sensitivity of the olfactory system is altered following blood-feeding. In this project, we are examining the hypothesis that regulation of specific olfactory and neurohumoral genes modifies the host-seeking behavior of female Aedes aegypti after blood-feeding.
The etiology of compulsive feeding behaviors including bulimia nervosa and binge eating disorder in humans is poorly understood. We propose that studying these important clinical conditions in a simpler genetic model system, the larva of the fruit fly Drosophila melanogaster, may shed new light on this important health problem. Fruit flies go through four distinct life stages: embryo, larva, pupa, and adult. While adult flies regulate their feeding according to hunger status and the circadian clock just like normal humans, the larva resembles a binge eater because it feeds continuously for nearly 72 hrs, eating 3-5 times its own weight in food. About 24 hrs before puparation, the larva abruptly leaves the food medium and stops eating. This highly stereotyped behavior provides an attractive experimental model to explore the neuronal mechanisms that drive and sustain continuous (compulsive) feeding. We hypothesize that continuous feeding in the Drosophila larva is a behavior accessible to genetic and pharmacological modulation. We are carrying out microarray analysis to identify candidate genes subject to regulation during continuous feeding. Using a genome-wide RNA interference (RNAi) screen, we hope to identify genes that modulate food intake. We will complement the RNAi screen with a small molecule screen that will look for compounds that reduce food intake. Finally, we will study the neuronal circuits modulating continuous feeding. Our long-term goal is to identify genes and neuronal circuits mediating the continuous feeding behavior of larvae and to prove that this compulsive-like behavior can be decreased by specific pharmacological interventions. We hope to illuminate common principles underlying the regulation of feeding behavior that will be applicable to parallel processes occurring in human patients suffering from compulsive eating disorders.
Funded by a grant from the Klarman Family Foundation Grants Program in Eating Disorders Research
Rhythmic cellular functions, such as the heartbeat and the timing of daily rhythms of activity and sleep, are regulated by the activity of cellular pacemakers. We are using genetic approaches to study the role of the Drosophila hyperpolarization- and cyclic nucleotide-gated ion channel, DmIh, in these processes. This project was a collaboration with Dr. Gareth Tibbs at Columbia University and Dr. Michael Nitabach at Yale University.
Insects have exquisitely sensitive olfactory systems that are tuned to food odors and pheromonal cues emitted by members of the same species. We have been studying the molecular mechanisms by which insect olfactory neurons respond to and discriminate among the numerous possible odors in the environment. Several years ago, we and others identified a divergent family of seven transmembrane domain receptors now known to be the insect odorant receptors (ORs). One member of the odorant receptor gene family, Orco, has the unique property that it is expressed in nearly all olfactory neurons. Therefore, each olfactory neuron in the fly is likely to express a conventional odorant receptor along with the co-receptor Or83b. Our recent work has shown that the insect odorant receptor is a heteromeric complex of the OR83b co-receptor with a conventional ligand binding odorant receptor. Orco is necessary and sufficient to target this OR/Orco complex to the ciliated dendrite of the olfactory sensory neuron. Together with our collaborator Dr. Kazushige Touhara and colleagues at the University of Tokyo, we investigated whether Orco has additional signaling functions beyond its role in ciliary trafficking. Our recent work provides strong evidence that the OR/Orco complex forms an odor-gated non-selective cation channel that does not depend upon G protein signaling. We are carrying out a large-scale in vivo structure-function analysis of Orco and the conventional odorant receptors to further probe the biology of these unusual membrane receptors. The goal is to map those domains that are necessary for the heteromeric association of the OR/Orco complex, domains necessary for trafficking, and residues that are necessary for odor signal transduction. We are particularly interested in discovering which residues may contribute to forming the ion-conducting pore. Going beyond conventional genetics, are using chemical biology to probe for small molecules that interfere with heterodimerization, trafficking, or signaling of OR/Orco complexes. Some of these compounds may be useful elements in a chemical strategy to block olfactory host-seeking behaviors in mosquitoes and other pest insects. These compounds may act as insect repellents that could be useful to control insect vectors that transmit human infectious diseases.
This work was funded in part by a grant to Richard Axel and L.B.V. from the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative
At an earlier developmental stage, Drosophila larvae display simple and robust olfactory-mediated chemotactic behavior controlled by 21 olfactory neurons expressing a repertoire of 25 odorant receptor genes. We have used a genetic approach to study odor coding in this simple animal by ablating specific sensory neurons and the receptors expressed in them, or by constructing animals with only a single functional olfactory neuron. By combining behavioral analysis with calcium imaging, we are attempting to understand the neural basis of olfactory behavior in this animal.
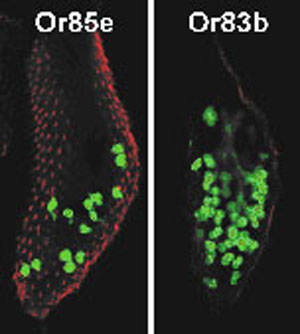
Using genetic labeling of olfactory sensory neurons expressing a given receptor, we generated a nearly complete map connecting odorant receptor expression to olfactory neuron convergence to antennal lobe glomeruli in adult Drosophila.
This work was supported in part by an individual F31 NRSA training grant from NIH/NIDCD to E.F. (5F31DC006795).
-
Laboratories 1/1